


Players and Forces
2017-09-27
Conceptual goals
- Know the main chemical forces that undergird life
- Understand the basis of the hydrophobic effect
- Know how the four main classes of biomolecules differ in these properties.
Skill goals
- Reason about the relative importance of different molecular forces in an aqueous environment
- Identify the ability to participate in different interactions from structure.
If $\Delta G < 0$ for $A \rightleftarrows B$, the reaction favors:
- $A$
- $B$
- Neither
- It depends
$B$
At equilibrium, $[A] > [B]$ for $A \rightleftarrows B$. This implies:
- $\Delta G ^{\circ}> 0$
- $\Delta G ^{\circ}< 0$
- Neither
$\Delta G > 0$
Explain what this equation means:
$\Delta G = \Delta H - T \Delta S$
Where a reaction ends up ($\Delta G$) depends on:
- Changes in the bonds formed (enthalpy, $\Delta H$)
- Change in the disorder (entropy, $T\Delta S$)
Road map
What are the important molecular interactions?
What about water?
Biological molecules and these interactions
What is the ratio of formed to unformed for covalent bonds at equlibrium?
$A + B \rightleftharpoons AB$
$K = \frac{[AB]}{[A][B]}$
Useful info: $R = 0.0083\ kJ \cdot mol^{-1} K^{-1}$, $T = 300\ K$
Ratio of formed to unformed is given by:
$\Delta G = -RTln(K)$
$K = \frac{[AB]}{[A][B]} = e^{--\Delta G/RT}$
$K = e^{--400/(300*0.0083)} = 6 \times 10^{69}$
Question:
The energy of a $Na^{+}$ + $Cl^{-1}$ bond in vacuum is $\approx -400\ kJ\cdot mol^{-1}$, but the book says ionic interactions are $\approx -70 kJ \cdot mol^{-1}$ in proteins. What is the origin of this difference?
Protip: water is 55 M and should never be ignored
Question:
The energy of a $Na^{+}$ + $Cl^{-1}$ bond in vacuum is $\approx -400\ kJ\cdot mol^{-1}$, but the book says ionic interactions are $\approx -70 kJ \cdot mol^{-1}$ in proteins. What is the origin of this difference?
Water competes the partners in an ion pair, this (effectively) weakens the bond.
Key point: The strength of an interaction depends on its context!
What happens if you jam a random molecule into water
Disrupts smooth handoff of one water to another
Solutes throw off a water molecule's "groove".
Fewer degrees of freedom means drop in entropy.
Drop in entropy makes the dissolving the compound less favorable.
$$\Delta G = \Delta H - T \color{red}{\Delta S}$$
Uber nerds:
$V_{old} = \frac{4}{3} \pi r_{old}^{3} $After merging, you have a sphere of $2 \times V_{old}$ with a radius:
$(2 \times V_{old}) = \frac{4}{3} \pi r_{new}^{3} $
$(2 \times \frac{4}{3} \pi r_{old}^3) = \frac{4}{3} \pi r_{new}^{3} $
$(2 \times r_{old}^3) = r_{new}^{3} $
$r_{new} = 2^{1/3} \times r_{old}$
Now compare surface areas:
$2 \times 4 \pi r_{old}^2 \stackrel{?}{>} 4 \pi r_{new}^{2}$
$2 \times r_{old}^2 \stackrel{?}{>} (2^{1/3} r_{old})^{2}$
$2 > {2}^{2/3}$
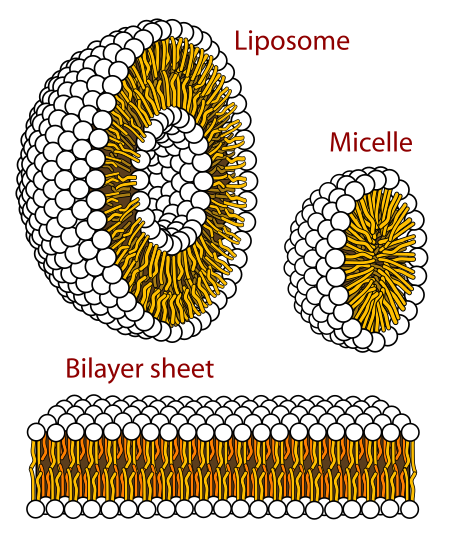
What happens when you create a molecule with both hydrophobic and polar character?
How hydrophobic is the molecule?
What interactions can it make?
What sorts of biological functions might it fulfill?
A
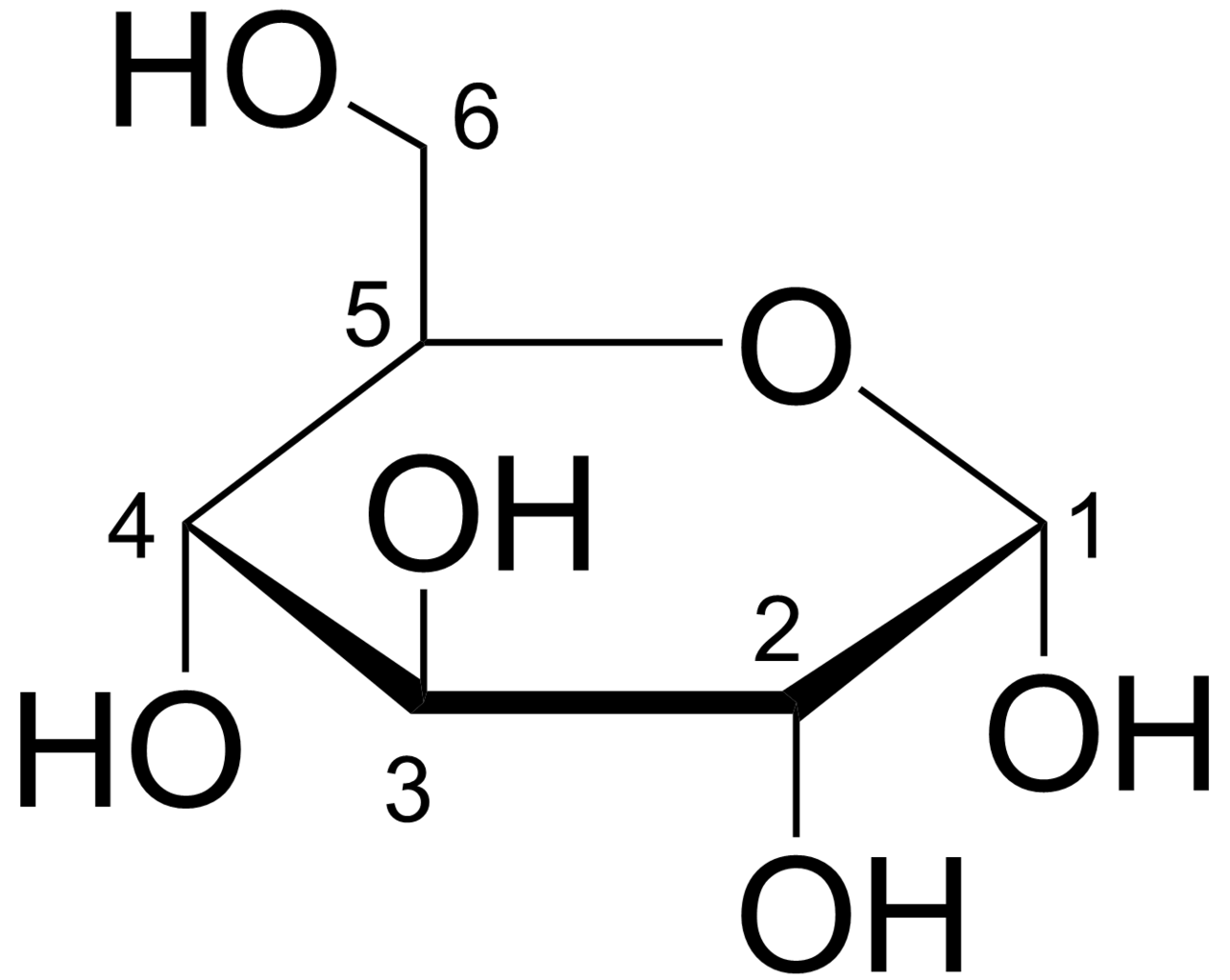
B
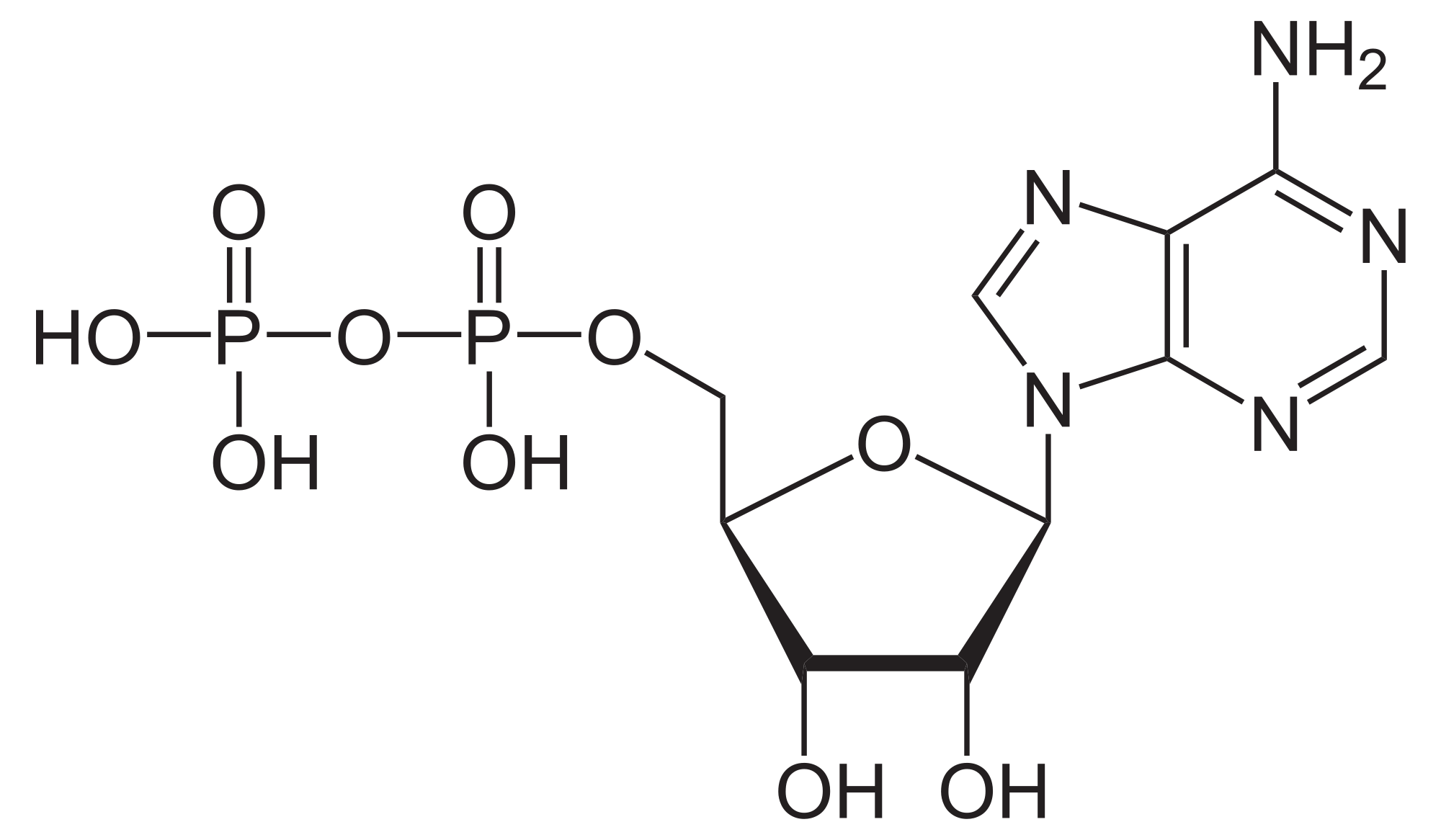
C
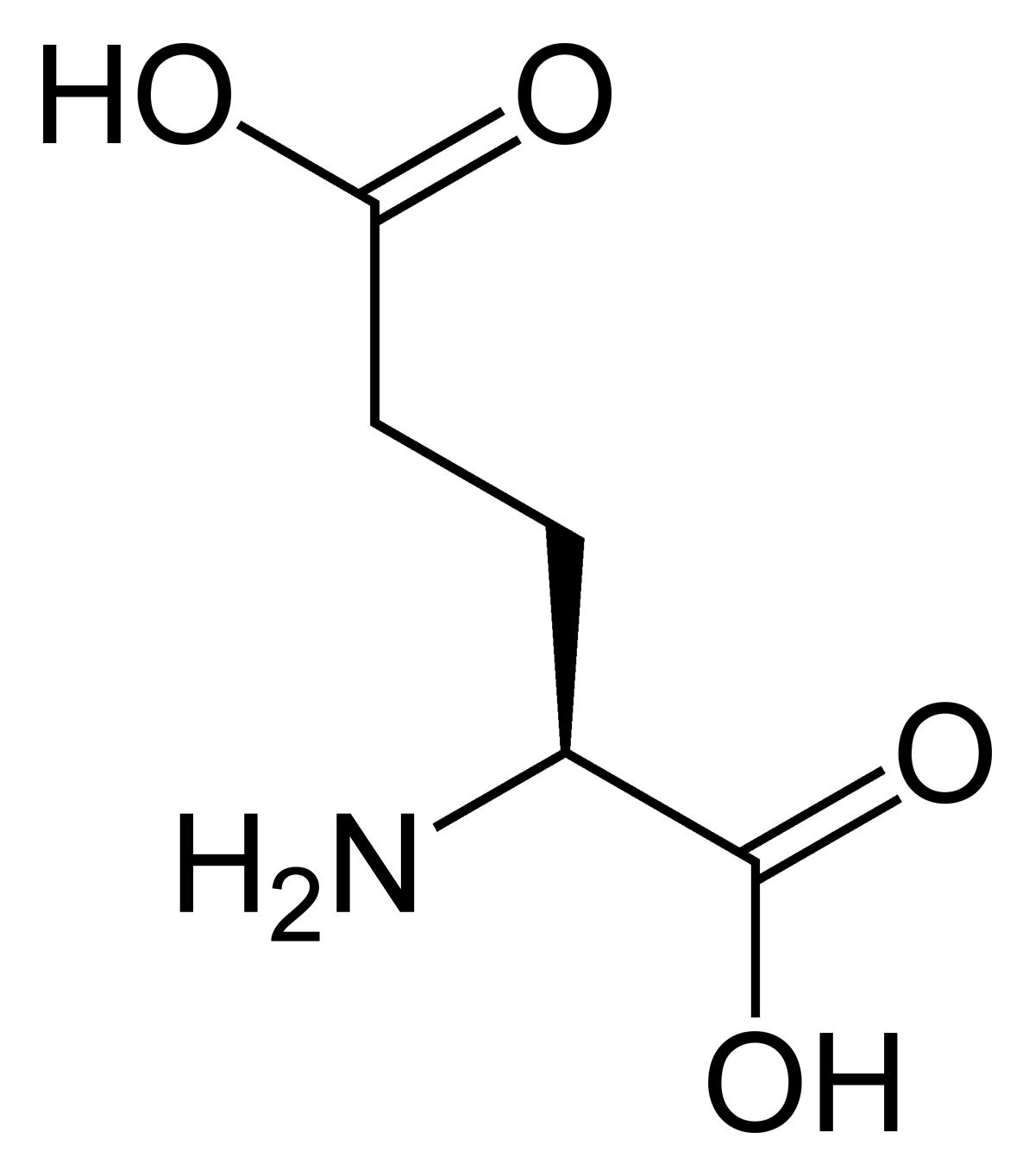
D
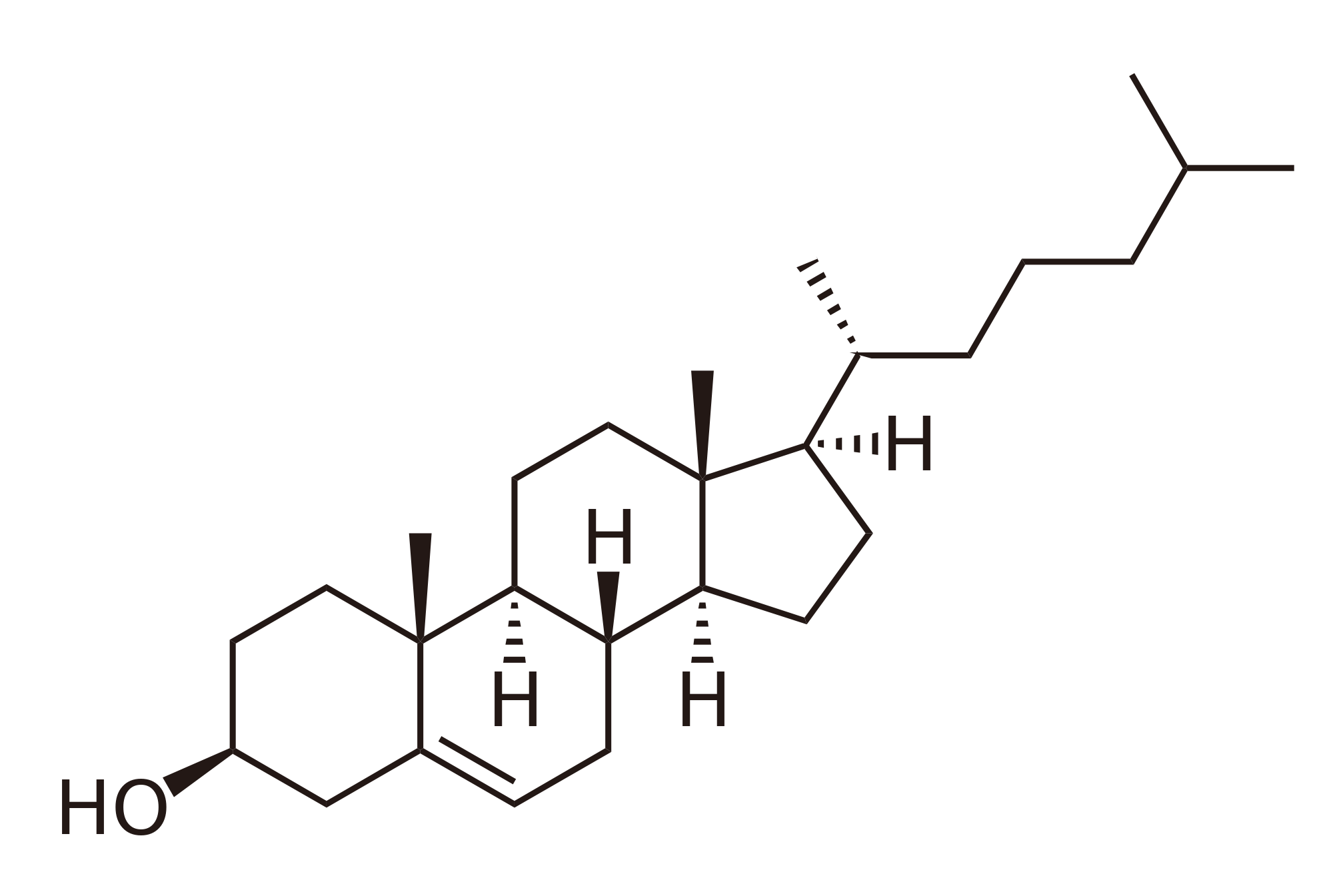
A

sugars
B

nucleic acids
C

amino acids
D

lipids
Summary
- Biomolecules are built (mostly) from CHNOPS
- These atoms participate in covalent, ionic, hydrogen-bond, and van der Waals interactions
- Water can change the net strength of these interactions
- The "hydrophobic effect" works to minimize the amount of solute surface interacting with water
- Most biomolecules are "amphipathic," leading to self-organization
- The four main molecular building blocks (sugars, nucleic acids, amino acids, and lipids) differ in these properties allowing them to fulfill different roles