Solutes throw off a water molecule's "groove".
Fewer degrees of freedom means drop in entropy.
Drop in entropy makes the dissolving the compound less favorable.
$$\Delta G = \Delta H - T \color{red}{\Delta S}$$
Hydrophobic effect
Causes molecules to cluster together to maximize $\Delta S$
What happens when you create an amphipathic molecule (has both hydrophobic and hydrophilic character)?
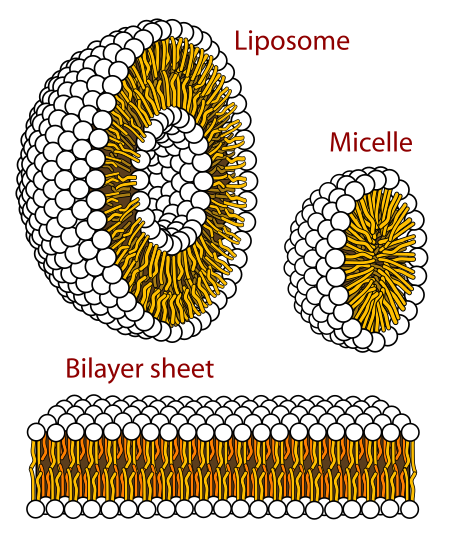
Big idea: making molecules that are both hydrophilic and hydrophobic can give large-scale order
wikimediaHow hydrophobic is the molecule?
What interactions can it make?
What sorts of biological functions might it fulfill?
A
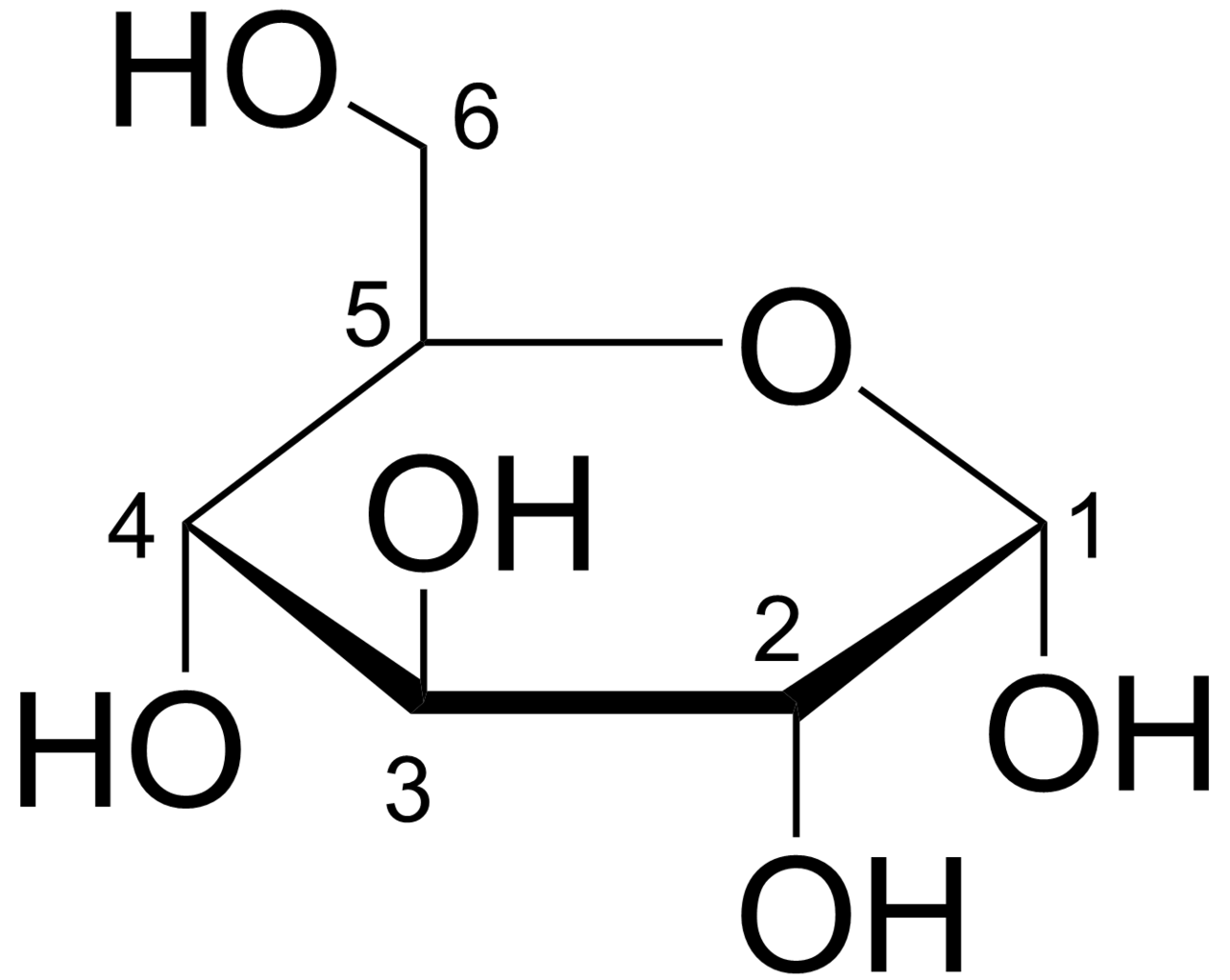
B
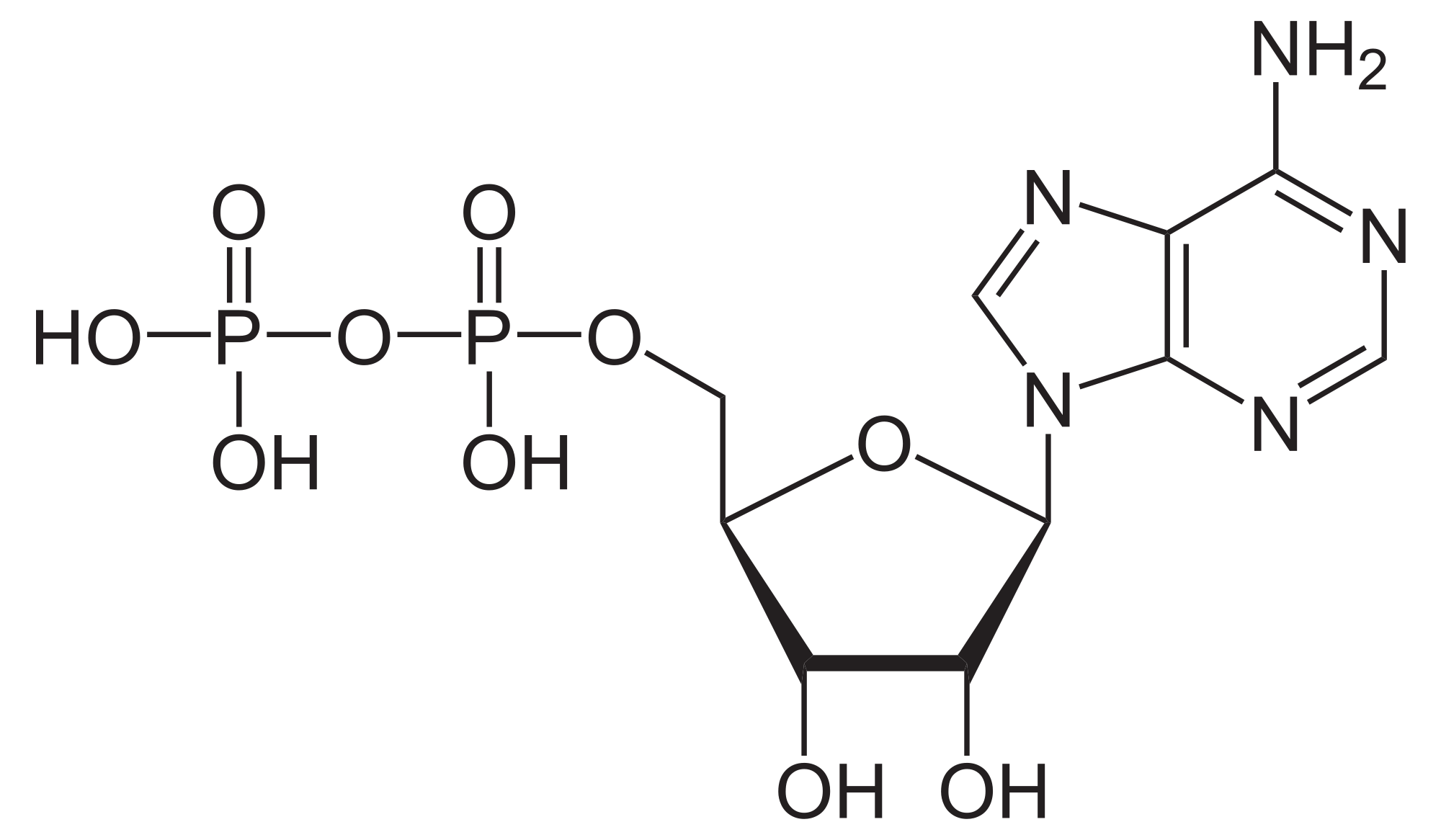
C
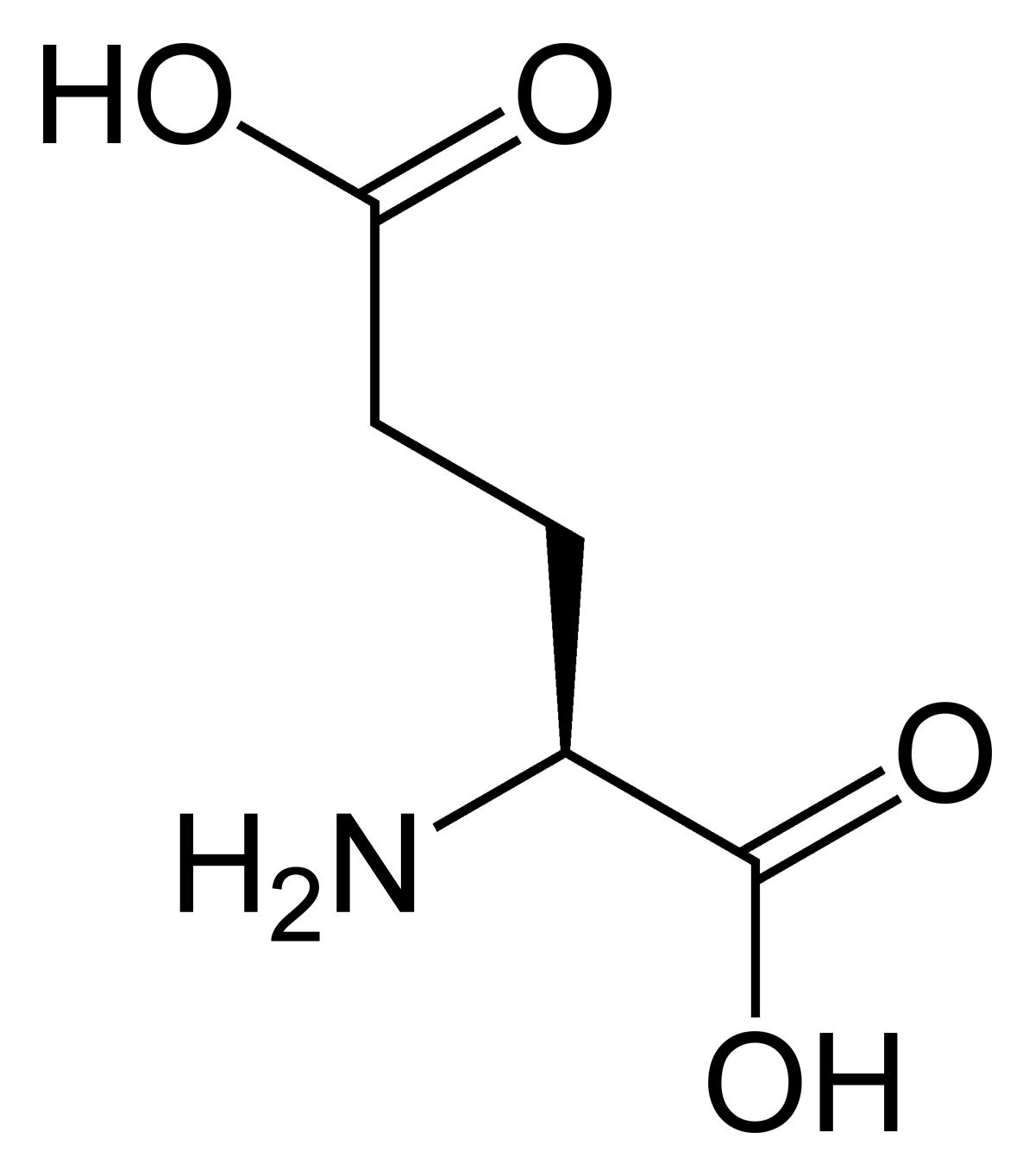
D
A

sugars
B

nucleic acids
C

amino acids
D

lipids
Summary
- Biomolecules are built (mostly) from CHNOPS
- These atoms participate in covalent, ionic, hydrogen-bond, and van der Waals interactions
- Water can change the net strength of these interactions
- The "hydrophobic effect" works to minimize the amount of solute surface interacting with water
- Most biomolecules are "amphipathic," leading to self-organization
- The four main molecular building blocks (sugars, nucleic acids, amino acids, and lipids) differ in these properties allowing them to fulfill different roles
Tiny Molecules with Large Effects
2017-09-29
Thinking about the pH scale makes me feel:

The pH scale was invented by Søren Peder Lauritz Sørensen working at Carlsberg Labs


Conceptual goals
- Understand that how pH is measured and treated by biochemists.
- Understand how proton concentration can link to other equilibria to bring about molecular change.
Skill goals
- Reason about equilibria.
- Manipulate the Henderson-Hasselbalch equation and reason about the charge state of macromolecules in different pH regimes.
PROTONATION STATE IS NOT THE SAME AS CHARGE STATE!
To convert protonation to charge, you need to know chemistry of the group. For example:
$\color{blue}{R-NH^{+}_{3}} \rightleftharpoons R-NH_{2} + \color{blue}{H^{+}}$
$R-COOH \rightleftharpoons \color{red}{R-COO^{-}} + \color{blue}{H^{+}}$
Summary
A few protons can have a huge effect in biochemistry by linking to other equilibria.
Biochemists usually describe proton tirations using the Henderson-Hasselbalch equation:
$$pH = pK_{a} + log \Big (\frac{[A]} {[HA]} \Big ) $$
The fractional protonation on a group is described by $\theta$:
$$\theta = \frac{1}{1 + 10^{pH - pK_{a}}}$$
Summary (continued)
The $pK_{a}$ is the $pH$ at which a titratable group is half-protonated.
To convert protonation to charge, you need to know chemistry of the group. For example:
$\color{blue}{R-NH^{+}_{3}} \rightleftharpoons R-NH_{2} + \color{blue}{H^{+}}$
$R-COOH \rightleftharpoons \color{red}{R-COO^{-}} + \color{blue}{H^{+}}$