Proteins Can Form Regular, Ordered Structures
2017-10-04
Conceptual goal
- Understand how the planar peptide bond and repeating polar backbone atoms lead to ordered secondary structure.
Skill goal
- Identify and reason about structural and functional roles of the three main types of secondary structure.
The amino acid sequence of a protein
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Primary structure
An assembly of multiple $\alpha$-helices and $\beta$-sheets
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Tertiary structure
An $\alpha$-helix or $\beta$-sheet
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Secondary structure
An assembly of multiple protein subunits
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Quaternary structure
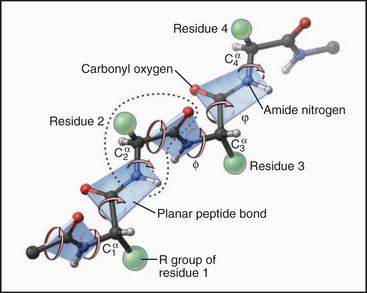
Peptide bonds are planes that rotate relative to one another
$\alpha$-helix
$\alpha$-helices
- Most common structural element in proteins
- Relatively rigid
- Regular pattern of nearby-in-sequence hydrogen bonds
- Lego-like on sides
$\alpha$-helices are lego-like
$\beta$-sheet
$\beta$-sheet
- Regular pattern of distant-in-sequence hydrogen bonds
- Can be either "parallel" or "anti-parallel"
- "Lego"-like on the sheet surface
Often form protein-protein interaction interfaces
Often form protein-protein interaction interfaces
$\beta$-sheets form amyloid fibers in neurological diseases
Almost any sequence can form an amyloid fiber.
- Why might most protein sequences be able to form a $\beta$-sheet?
- Amyloid fibers, once started, keep sucking in new proteins. Why might this be?
Almost any sequence can form an amyloid fiber.
- Almost any protein sequence can form a $\beta$-sheet because the sidechains point out into solvent (no steric hinderance)
- Amyloid fibers, once started, keep sucking in new proteins because they have "open-ended" edges that can hydrogen bond
Loops
Loops
- Connects other elements of secondary structure
- Relatively dynamic/floppy
- Irregular structures and hydrogen bonds
Loops connect $\alpha$-helices and $\beta$-sheets
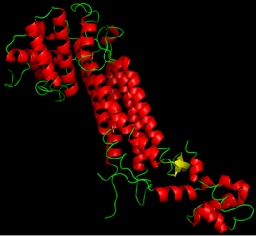
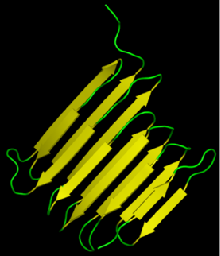
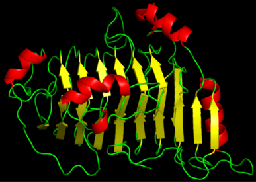

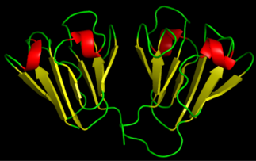
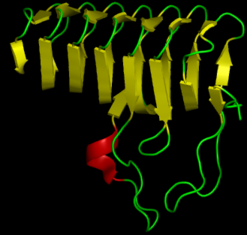
Proteins are organized hierarchically from pieces of secondary structure that pack together
Discuss: why does a protein sequence take a particular structure?
Optimizes all possible interactions that can be formed by polypeptide.
Hydrogen bonds
Hydrogen bonds
Ionic bonds
Disulfide
Hydrophobic effect
Color spectrum from Nonpolar to Polar
Hydrophobic effect
Color spectrum from Nonpolar to Polar
Proteins are dynamic
$ps$ to $ns$ dynamics ($10^{-12}\ to\ 10^{-9}\ s$)
$\mu s$ to $ms$ dynamics ($10^{-6}\ to\ 10^{-3}\ s$)
Summary
- Planar peptide bond leads to repeating, opposite donors and acceptors
- These donors and acceptors form regular, repeating secondary structures
- These secondary structures pack together to assemble a full protein structure
- These structures, while ordered, are still highly dynamic
Final puzzle
Why do all of these secondary structure hydrogen bonds form if they can just as easily form with water?