First midterm: tonight at 7 pm in McKenzie 240C
Covers weeks 1-3 (lectures 1-9, homework 1-3, lab 1-3).
Bring a calculator
The exam is closed note; however, there is a data sheet on the back page with important formulas and constants.
You may use a calculator on the exam, but no Internet-enabled device (cell phone, tablet, laptop, etc).
Enzymes: mechanism of serine protease
2017-10-18
Enzymes work by:
- Destabilizing the substrate
- Increasing the effective temperature of the reaction
- Stabilizing the transition state
Stabilizing the transition state
A small decrease in "activation" energy ($\Delta \Delta G$) gives:
- A linear increase in rate ($\frac{k_{enz}}{k_{non}} \sim \Delta \Delta G$)
- A quadratic increase in rate ($\frac{k_{enz}}{k_{non}} \sim \Delta \Delta G^{2}$)
- An exponential increase in rate ($\frac{k_{enz}}{k_{non}} \sim e^{\Delta \Delta G}$)
- A sinusoidal increase in rate ($\frac{k_{enz}}{k_{non}} \sim sin(\Delta \Delta G$))
An exponential increase in rate ($\frac{k_{enz}}{k_{non}} ~ e^{\Delta \Delta G}$)
Conceptual goals
- Understand the chemical mechanism of serine protease.
- Understand the core ways enzymes speed up rates: orient substrates, sequester from water, interact with transition state, do sequential low barrier chemical steps.
Skill goals
- Trace/explain the electron "arrows" in this mechanism.
- Reason about the effects of mutations on its activity.
Peptide bond is a hydrolysis reaction
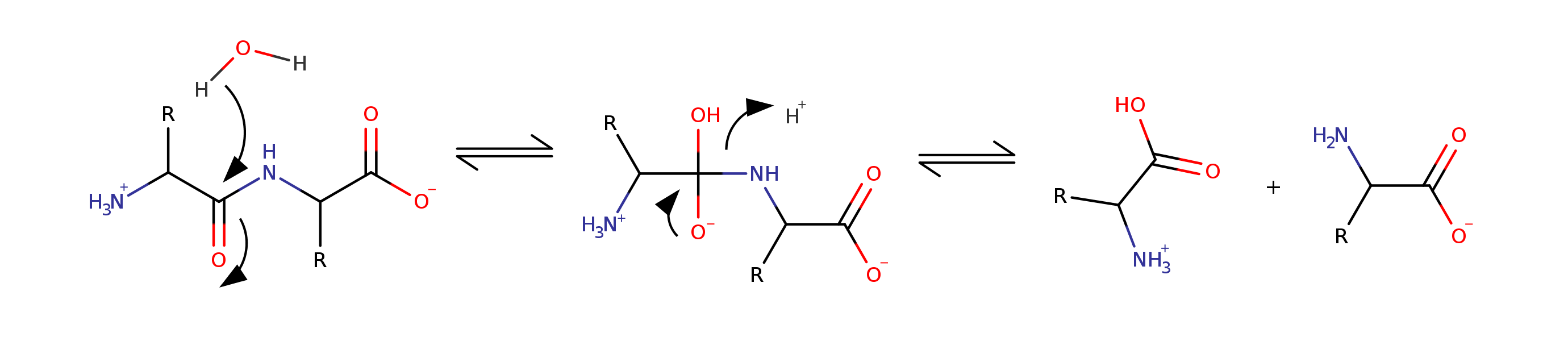
rate constant = $k = 1.5 \times 10^{-9}\ s^{-1}$
$k = 0.05\ years^{-1}$. That means 20 years to break a bond!
The "Active Site" is where the chemistry happens
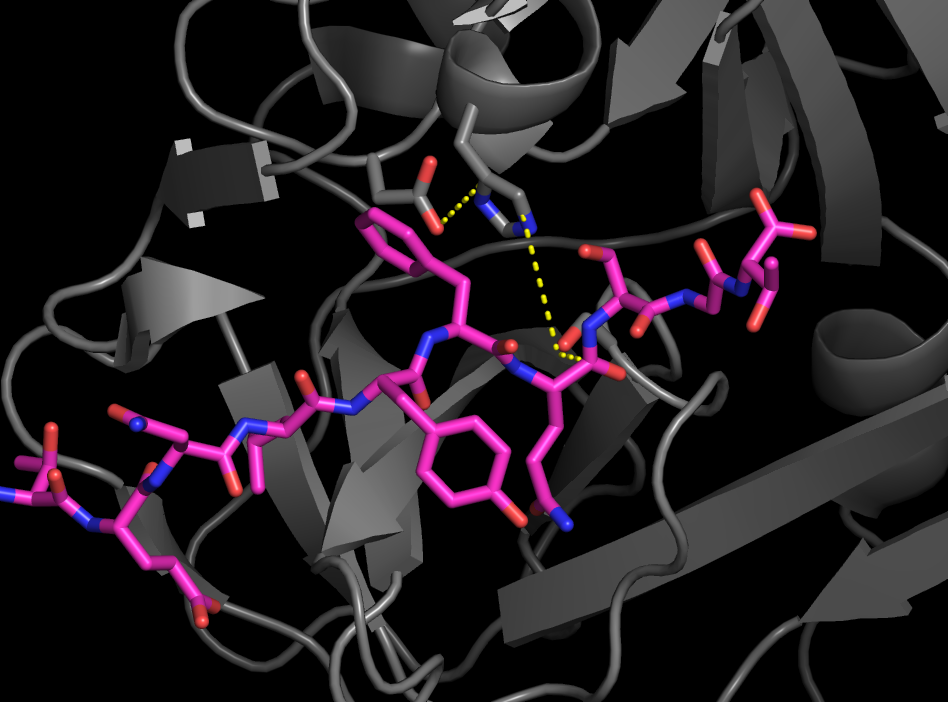
Made of Asp-His-Ser catalytic triad
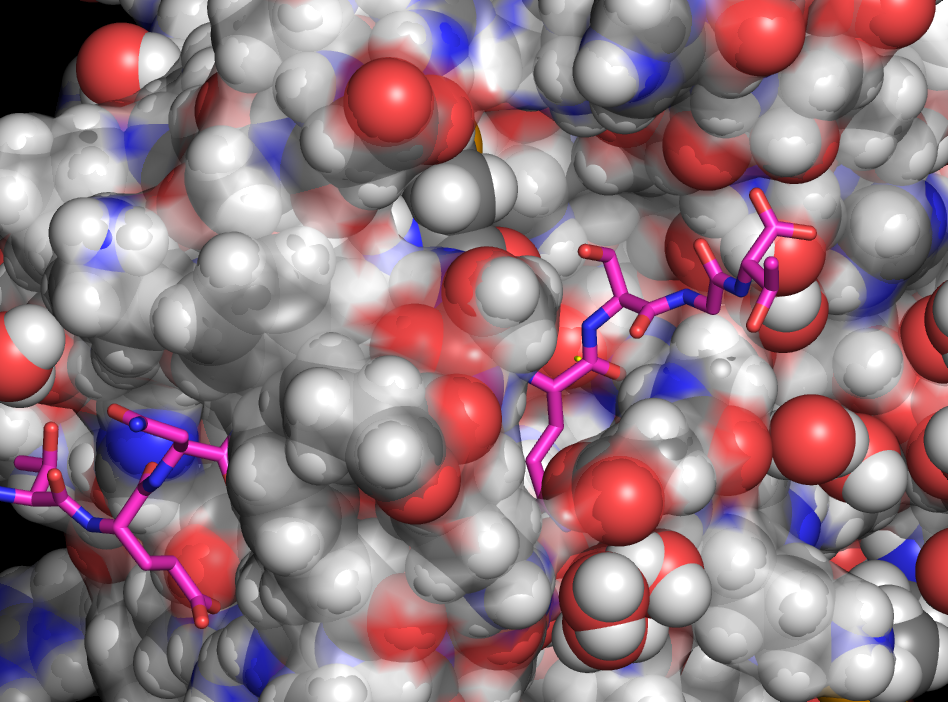
Active site pulled away from water
The catatlyic triad is made of Asp-His-Ser
Step 1: $R-S^{-}$ attack on carbonyl
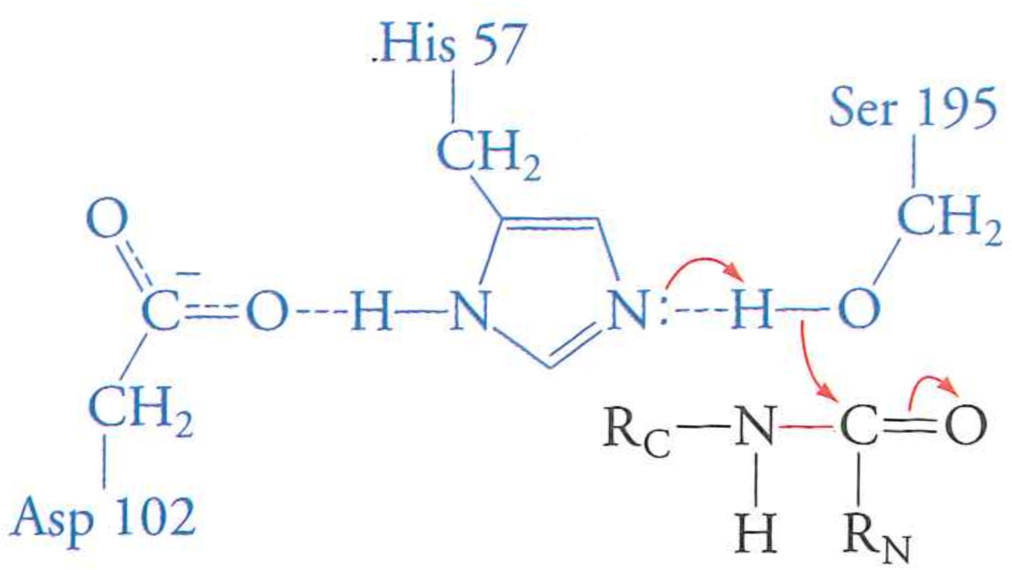
Puzzles:

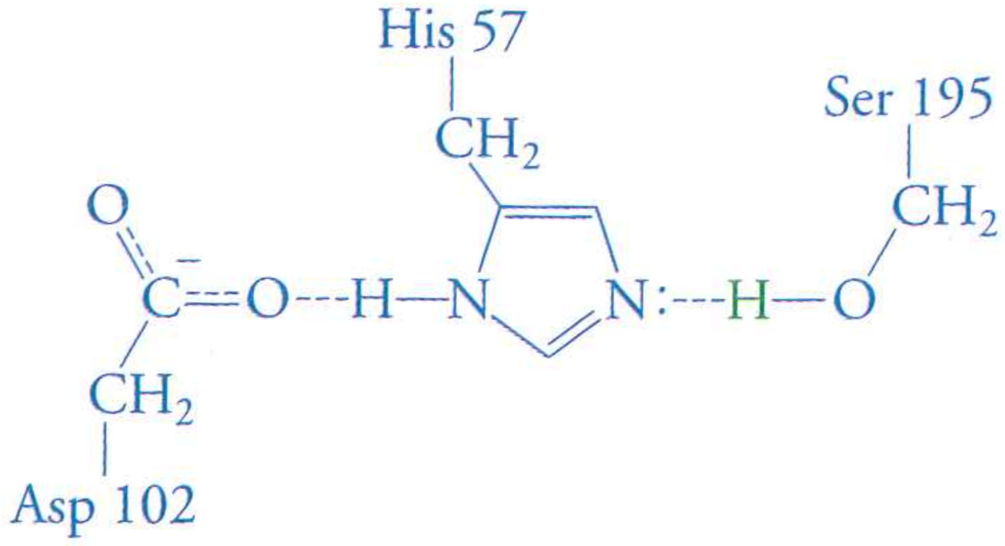
Aspartic acid away from water desperately needs to fulfill its interactions
The negative aspartic acid can form a (quasi) ionic bond if Histidine protonates and becomes positive
His pulls on the Ser proton, lowering its $pK_{a}$ to near neutral.
Big ideas
1. Enzymes orient chemical groups so they're primed to do chemistry.
2. Enzymes activate polar atoms by pulling them away from water.
Step 1: $R-S^{-}$ attack on carbonyl

This creates a negatively charged tetrahedral intermediate
Oxyanion hole stabilizes tetrahedral intermediate by interacting with negative oxygen
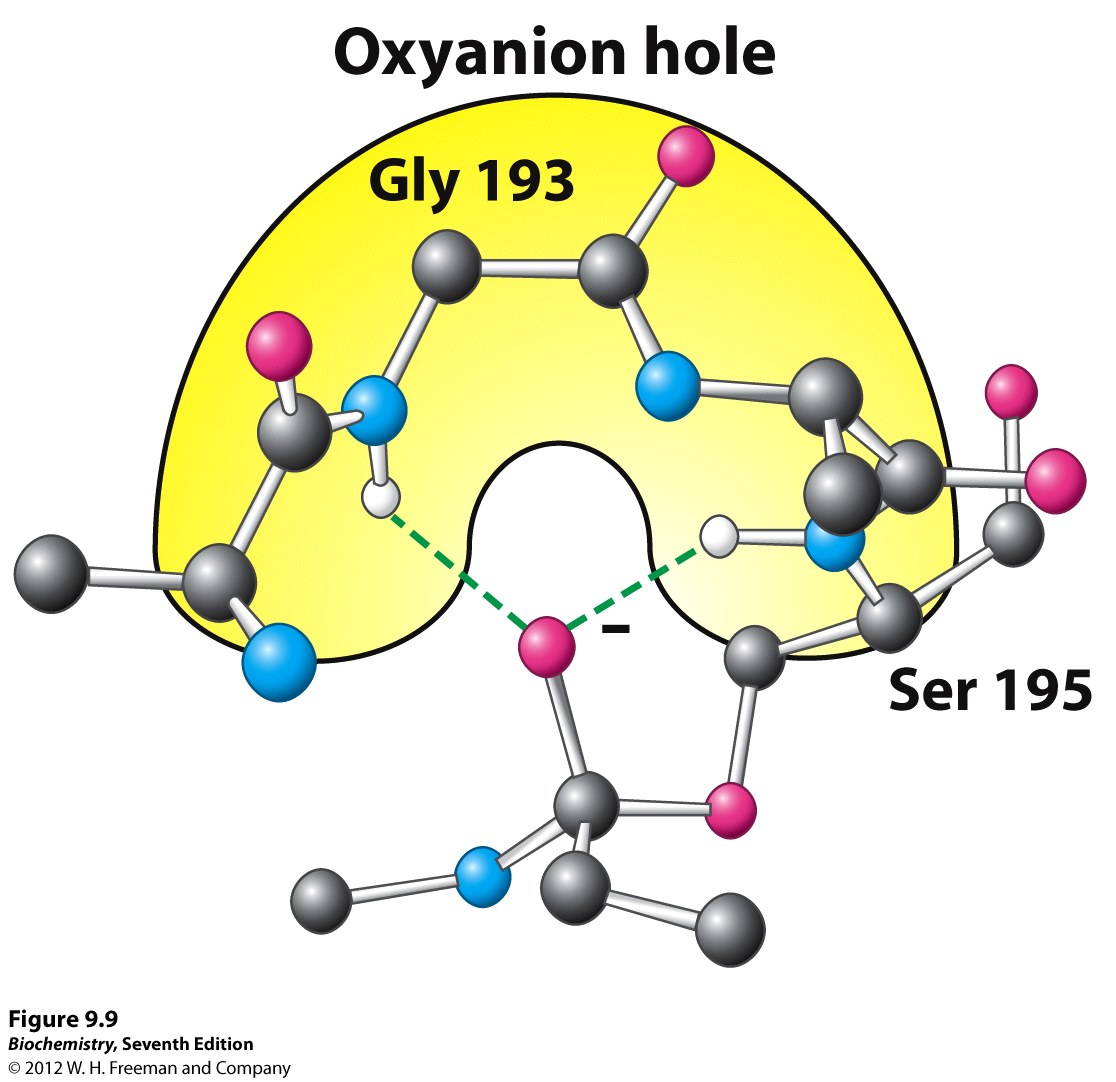
Credit: oregonstate.edu
Big ideas
1. Enzymes orient chemical groups so they're primed to do chemistry.
2. Enzymes activate polar atoms by pulling them away from water.
3. Enzymes interact with/stabilize transition state.
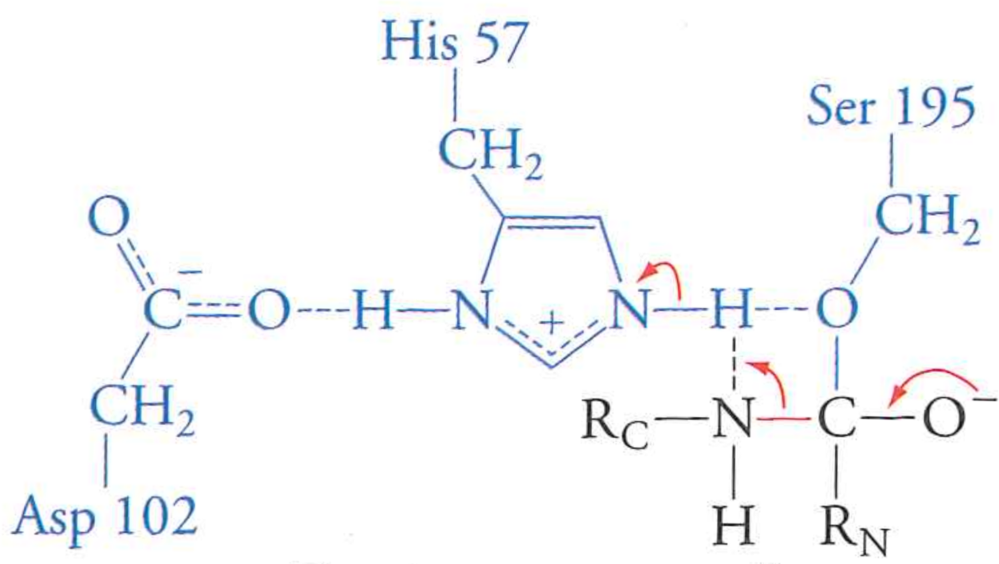
Step 2: break the peptide bond


Electrons flow from negative oxygen, but rather than breaking new peptide-serine bond, break peptide bond to satisfy electron-poor histidine
Newly cleaved C-terminal bit leaves the active site by diffusion. Remainder of peptide covalently attached to Serine.
Step 2: break the peptide bond

Step 3: newly cleaved C-terminal peptide diffuses away
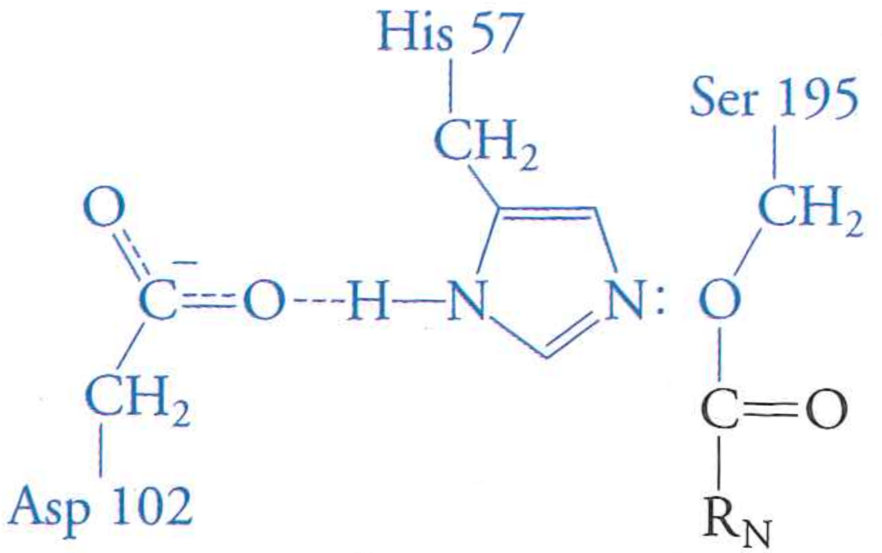
Are we done yet?
No. Enzyme still has peptide covalently attached.
Step 3: newly cleaved C-terminal peptide diffuses away

Step 4: water enters the binding site and attacks peptide carbonyl
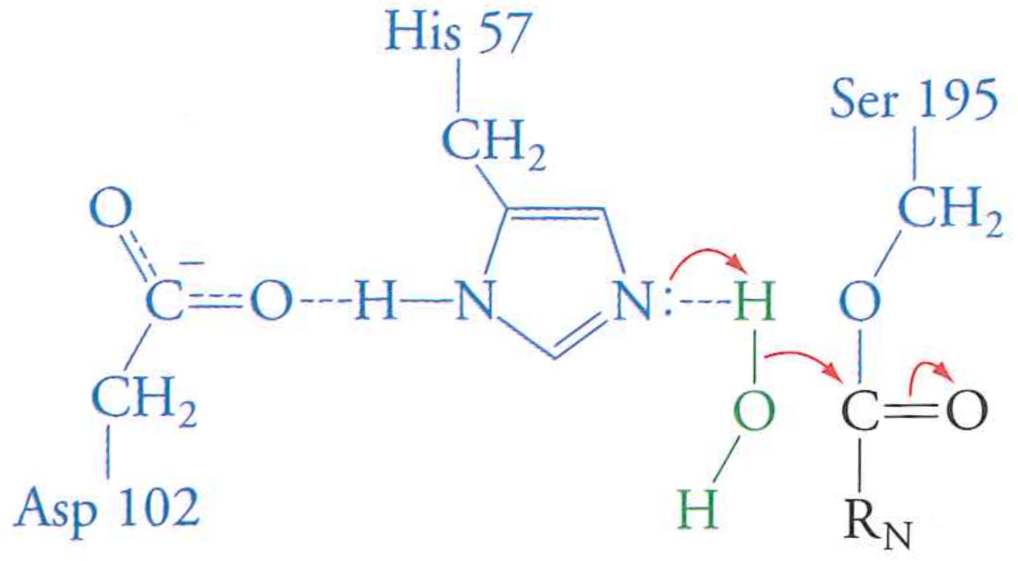

Water is "activated" by that needy histidine in the same way as Serine
Step 4: water enters the binding site and attacks peptide carbonyl

Step 5: electrons flow from charged oxygen and break peptide-serine bond
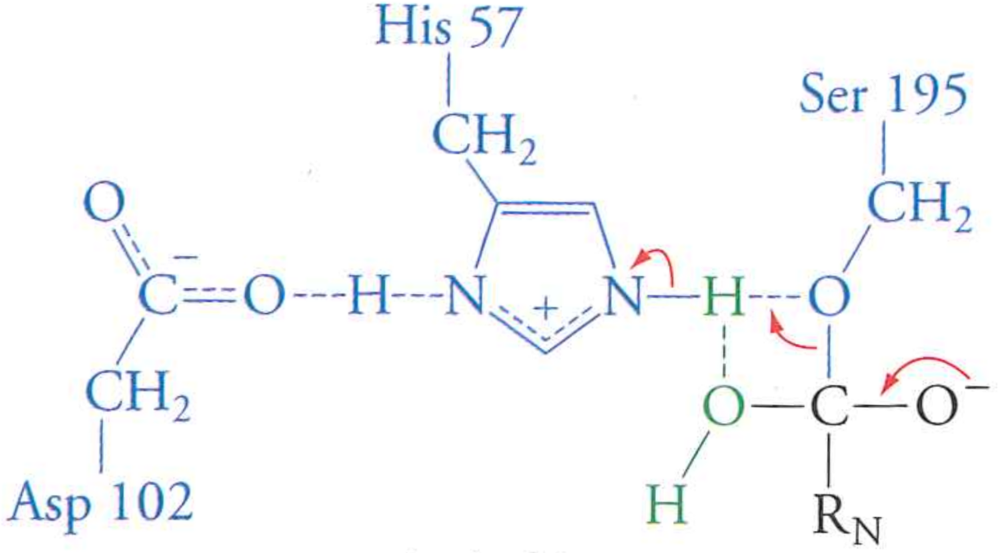
Step 6: newly cleaved peptide diffuses out of active site, enzyme restored

Big ideas
1. Enzymes orient chemical groups so they're primed to do chemistry.
2. Enzymes activate polar atoms by pulling them away from water.
3. Enzymes interact with/stabilize transition state.
4. Enzymes do hard chemistry by breaking it into multiple, low-barrier steps
Specifcity is achieved by polar interactions outside of active site
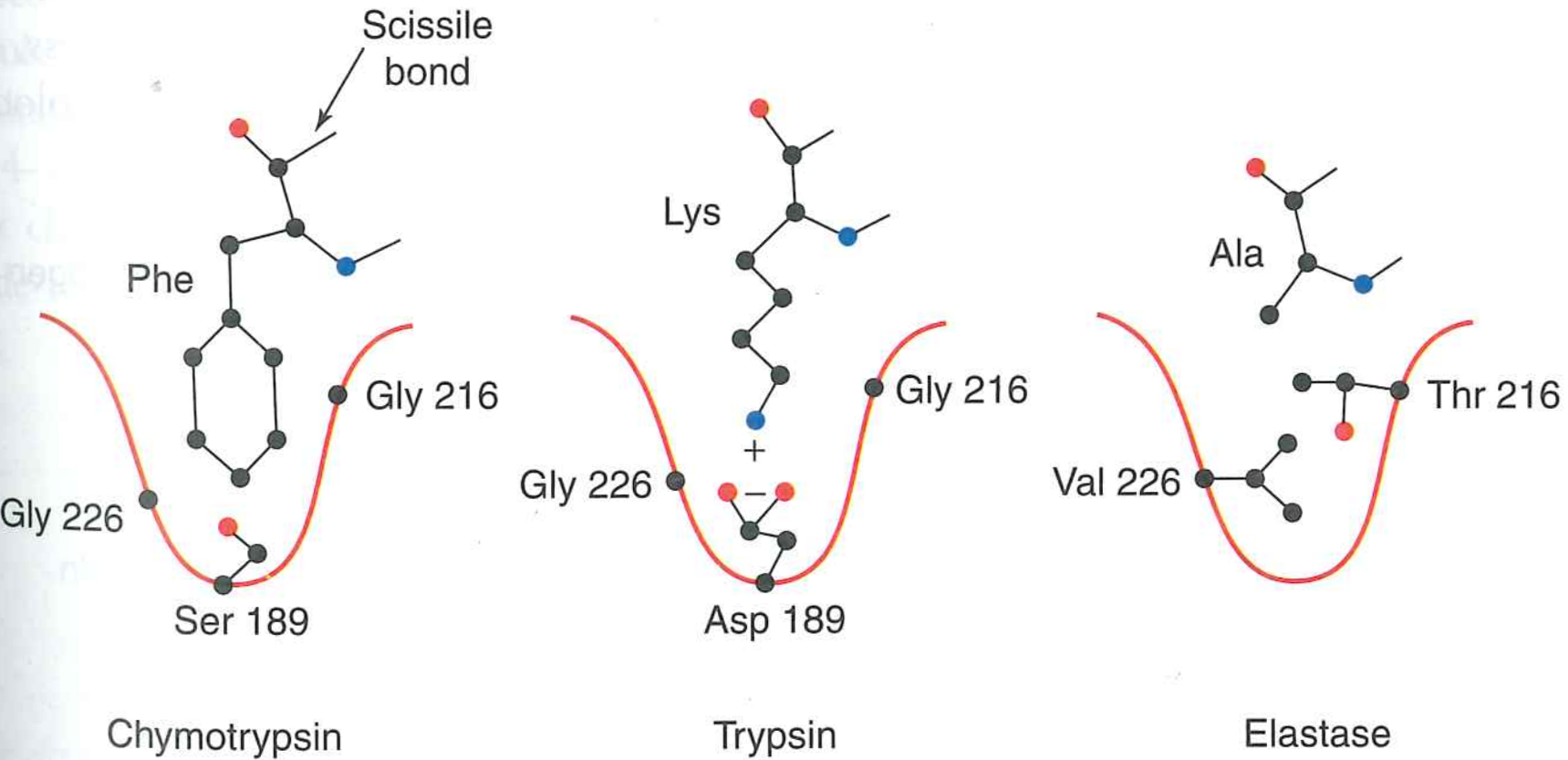
Tobacco Etch Virus protease recognizes ENLYFQG
Big ideas
1. Enzymes orient chemical groups so they're primed to do chemistry.
2. Enzymes activate polar atoms by pulling them away from water.
3. Enzymes interact with/stabilize transition state.
4. Enzymes do hard chemistry by breaking it into multiple, low-barrier steps
5. Specificity is encoded by interactions near the active site
Exact active site geometry has evolved many times
Class review
Let's have a different person answer each question
Step 0: primed catalytic triad

Why is the Asp-His hydrogen bond strong? Both are away from water in the active site
Step 0: primed catalytic triad

How does this change the strength of the His-Ser H-bond? Negative Asp stabilizes positive His, increasing affinity of His for proton
Step 1: $R-S^{-}$ attack on carbonyl

How can a Ser ($pK_{a}\ 17$) deprotonate at $pH\ 7$? The needy His "pulls" on the Ser proton and thus lowers the $pK_{a}$ of Ser.
Step 1: $R-S^{-}$ attack on carbonyl

Is this activated Ser a better or worse nucleophile than water? Much better
Step 2: break the peptide bond

How does the enzyme stabilize the negative tetrahedral intermediate? Favorable polar interactions with negative charge (oxyanion hole)
Step 2: break the peptide bond

Why does the peptide bond break rather than the Ser-peptide bond? Histidine is now formally positive and is now more attractive to electrons than the Ser
Step 3: newly cleaved C-terminal peptide diffuses away

Step 4: water enters the binding site and attacks peptide carbonyl

What business does a water molecule have in a (relatively) hydrophobic active site? 1. Water is 55 M. 2. lone pair on histidine nitrogen needs an acceptor.
Step 5: electrons flow from charged oxygen and break peptide-serine bond

Step 6: newly cleaved peptide diffuses out of active site, enzyme restored

Big ideas
1. Enzymes orient chemical groups so they're primed to do chemistry.
2. Enzymes activate polar atoms by pulling them away from water.
3. Enzymes interact with/stabilize transition state.
4. Enzymes do hard chemistry by breaking it into multiple, low-barrier steps
5. Specificity is encoded by interactions near the active site