Allostery
2017-10-27
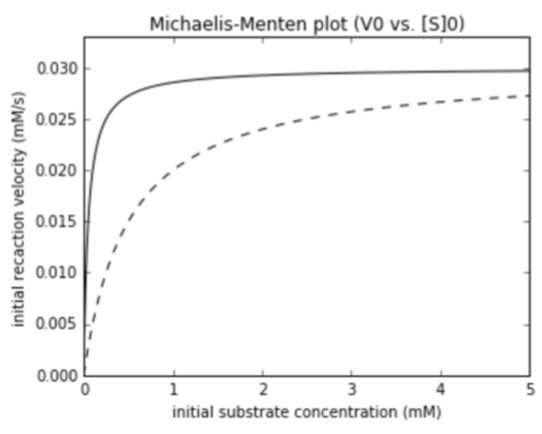 $K_{M}$, competitive
$K_{M}$, competitive
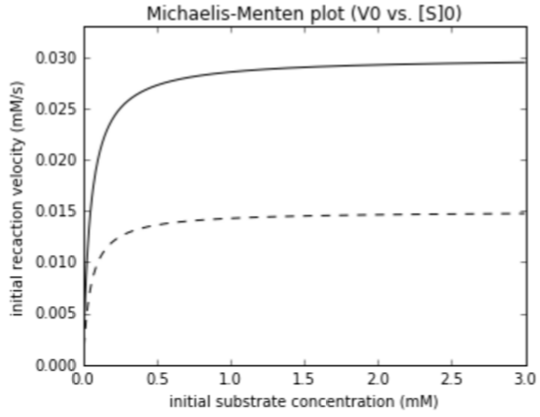 $V_{max}$, noncompetitive
$V_{max}$, noncompetitive
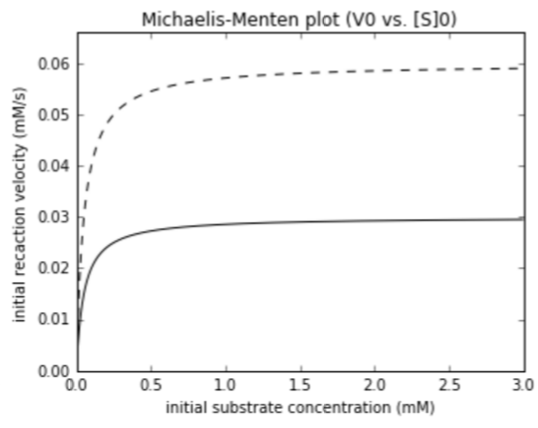 $V_{max}$, activator
$V_{max}$, activator
Competitive inhibitor
- Inhibitor competes with substrate for same site
- This raises $K_{M}$: lower apparent affinity for substrate because it has to compete
- No effect on $V_{max}$. If you add enough substrate, you swamp out competitor
- Inhibitor is chemically similar to the substrate
- Example: Ethanol competes with methanol for alcohol dehydrogenase, lowering the rate of formaldehyde production.
Noncompetitive inhibitor
- Binds distant from the active site, altering active site to turn off activity
- This leads to a drop in $V_{max}$. (lower $[E]_{T}$: Less of the enzyme is in the active form.)
- No effect on $K_{M}$. What active enzyme is around has exact same affinity for substrate
- The inhibitor need not have any chemical similarity to the substrate
Example noncompetitive inhibitor
Conceptual goals
- Understand that binding at one site in a protein can alter activity at another site
- This "allostery" arises because the "allosteric effector" interacts with one conformation, but not the other.
- Understand how this applies to hemoglobin (BPG).
Skill goals
- Determine how binding at on site affects activity at the other
- Predict the effects of adding allosteric effectors to a system of equilibria.
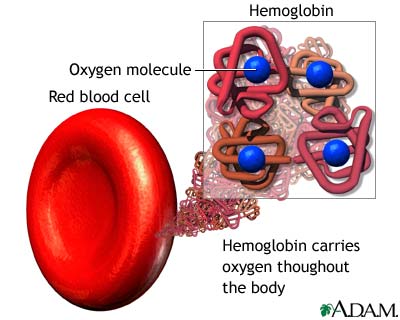
Hemoglobin transports $O_{2}$ from the lungs to tissues
stephaniefuturedoc
Hemoglobin is a tetramer four proteins
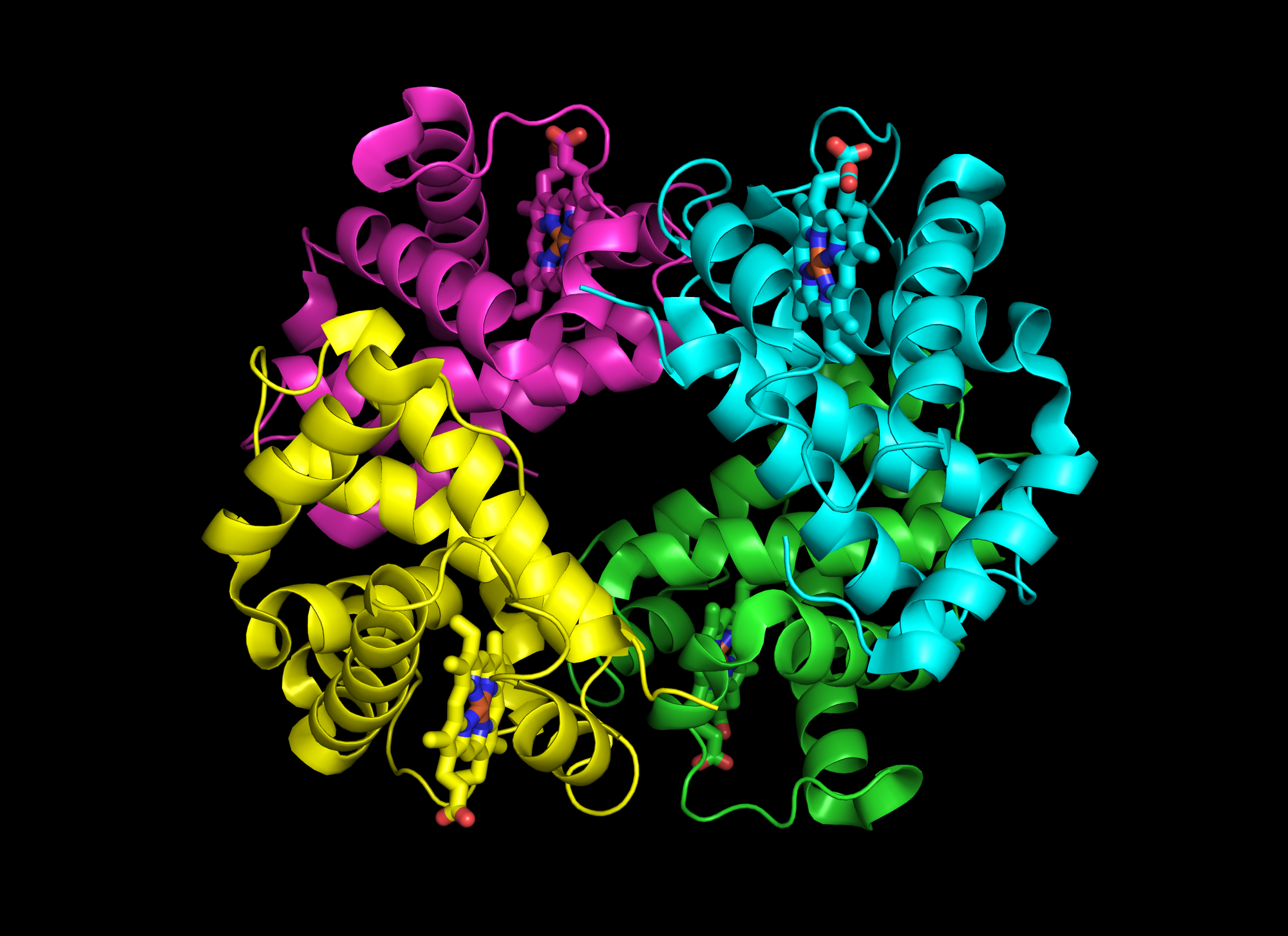
This is through linked equilibria
$E_{active} + I \rightleftarrows E_{inactive} + I \rightleftarrows E_{inactive} \cdot I$
$[E]_{active} = [E]_{T}\theta_{active}$
$\theta_{active} = \frac{[E_{active}]}{[E_{active}] + [E_{inactive}] + [E_{inactive}\cdot I]}$
What are the ingredients you would need for allostery?
Ingredients:
- Two different binding sites recognizing different things
- Equilibrium between two (or more) protein shapes
- Different "activities" (functions, properties, etc.) of each shape
- Binding to one shape, but not the others
Summary
Allostery is when binding at one site in a protein alters activity at another site
- Two different binding sites recognizing different things
- Equilibrium between two (or more) protein shapes
- Different "activities" (functions, properties, etc.) of each shape
- Binding to one shape, but not the others
Noncompetitive inhibitors
BPG allosterically regulates $O_{2}$ binding in hemoglobin