Energy Transduction
2017-11-06
This is a primary reaction underlying metabolism (glucose oxidation):
$C_{6}H_{12}O_{6} + 6O_{2} \rightarrow 6CO_{2} + 6H_{2}O$
What type of reaction is this?
Combustion reaction
 publicdomainimages.net
publicdomainimages.net
How do living things avoid burning up?
Break combustion into many steps
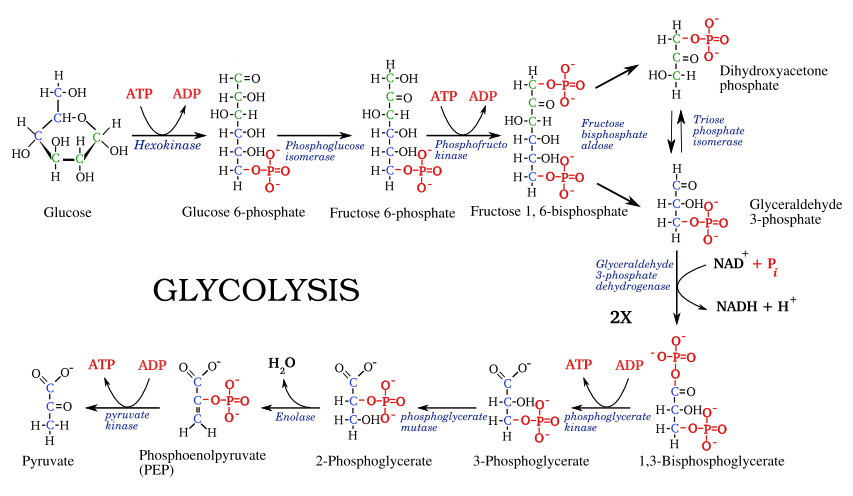
LibreTexts
Conceptual goals
- Understand the basics of REDOX chemistry as they apply to biological systems.
- Energy transduction requires capturing reaction outputs--like a turbine on a generator.
- Understand that ATP (and ilk) acts as energy currency and that NADH (and ilk) act as electron currency.
Worksheet
$O_{2} + 2H_{2} \rightarrow 2H_{2}O$
Products:
- $1 \times O=O$
- $2 \times H-H$
Reactants:
- $4 \times O-H$
Estimating $\Delta H^{\circ}$
$(O=O) + (2\times H-H) \rightarrow (4 \times O-H)$
$(498) + (2\times 436) \rightarrow (4 \times 464)$
$1370 \rightarrow 1856$
$1856 - 1370 = 486$
$\Delta = 486\ kJ\cdot mol^{-1}$
This is exothermic, as it is more unfavorable to break product bonds than reactant bonds
Why is this reaction exothermic?
- In $H_{2}O$, electrons are shared between $O$ and $H$.
- This is favorable because the electronegative $O$ can dominate the $H$ and "own" the electrons.
- For $H_{2}$ and $O_{2}$, electrons are shared between atoms of equal electronegativity.
- Electrons are not closest to most electronegative atoms.
Is $H_{2}$ oxidized or reduced in this reaction?
Oxidized. Hydrogens lose electrons (which are now "owned" by oxygen)
NOTE: REDOX reactions are always paired $H_{2}$ was oxidized, $O_{2}$ was reduced
If an $ADP+P_{i}\rightarrow ATP$ takes $30.5\ kJ\cdot mol^{-1}$, how many $ATP$ could you form with this reaction?
$486/30.5 = 15.9 ATP$.
$ATP \rightarrow ADP + P_{i}$
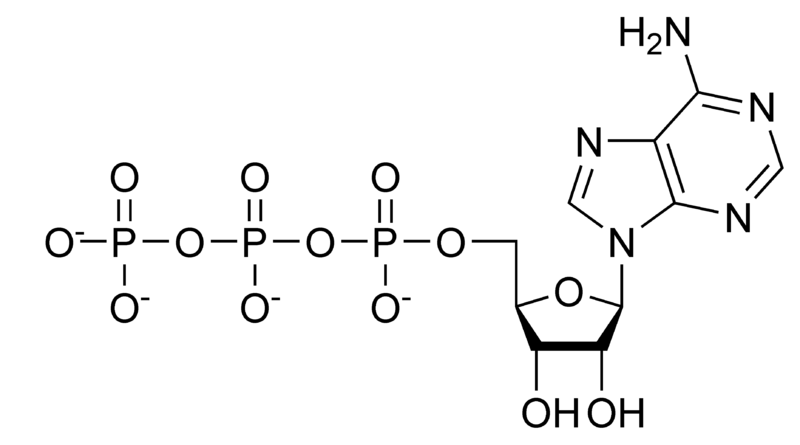
adenosine triphosphate
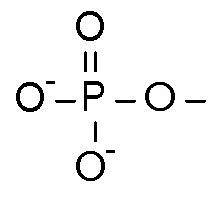
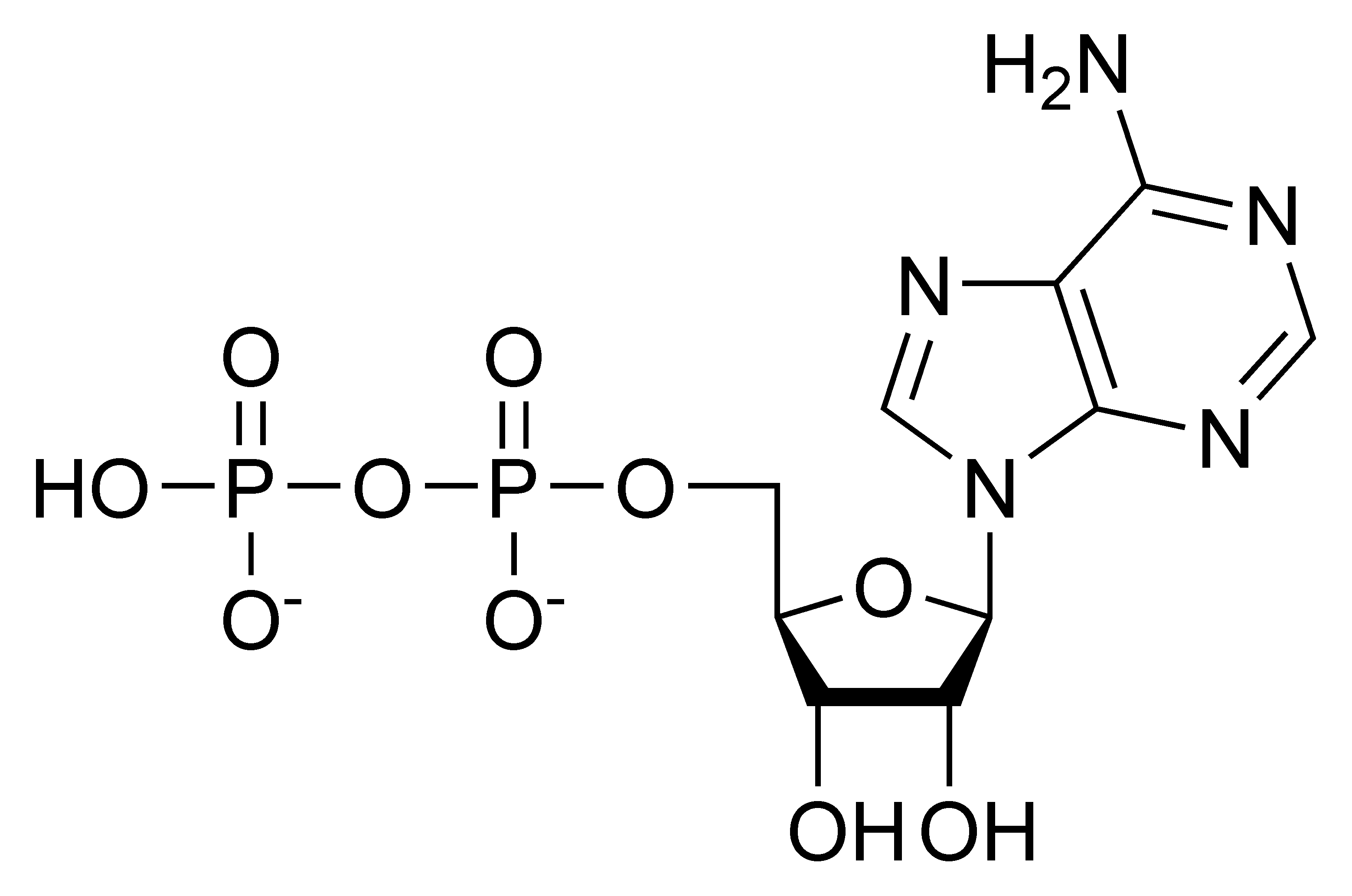
phosphate + adenosine diphosphate
ATP
- ATP is the energy currency of the cell
- Useful unit of energy
- "Exchangable" for other units of energy
- Easy to transport etc.
- Breaking an ATP phosphate bond yields a useful amount of energy ($30.5\ kJ\cdot mol^{-1}$ at $pH\ 7$)
- Favorable energy comes from electrostatic repulsion, resonance, and entropy
$C_{6}H_{12}O_{6} + 6O_{2} \rightarrow 6CO_{2} + 6H_{2}O$
electrons are transferred from $C_{6}H_{12}O_{6}$ and $O_{2}$ to $CO_{2}$ and $H_{2}O$
To break up reaction into steps, we need way to temporarily hold on to electrons
$NAD^{+} + H^{-} \rightarrow NADH$
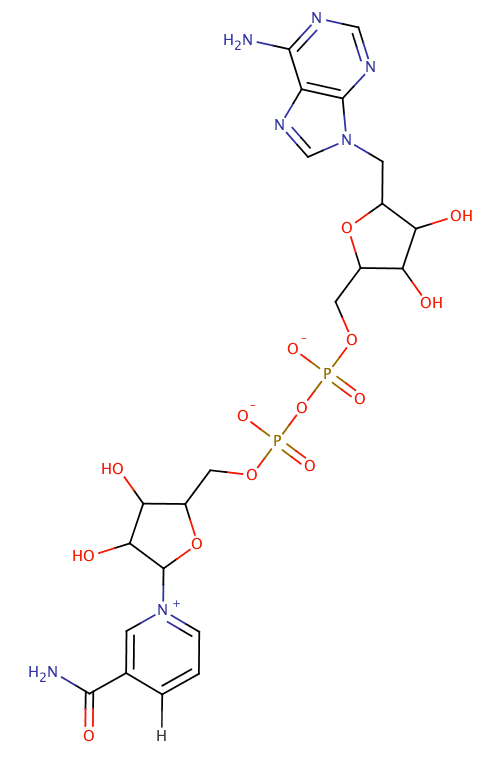
nicotinamide adenine dinucleotide
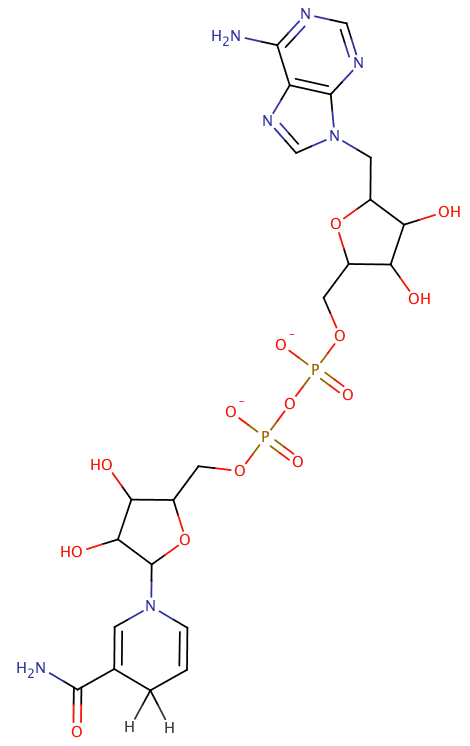
nicotinamide adenine dinucleotide phosphate
NADH
- NADH is the electron currency of the cell
- Can exist as $NAD^{+}$ (with resonance stabilized $N^{+}$)
- Or pick an $H^{-}$ (i.e. $H:$) and be NADH
- Its ability to be reduced allows it to oxidize biomolecules
- This allows for controlled $e^{-}$ transfer
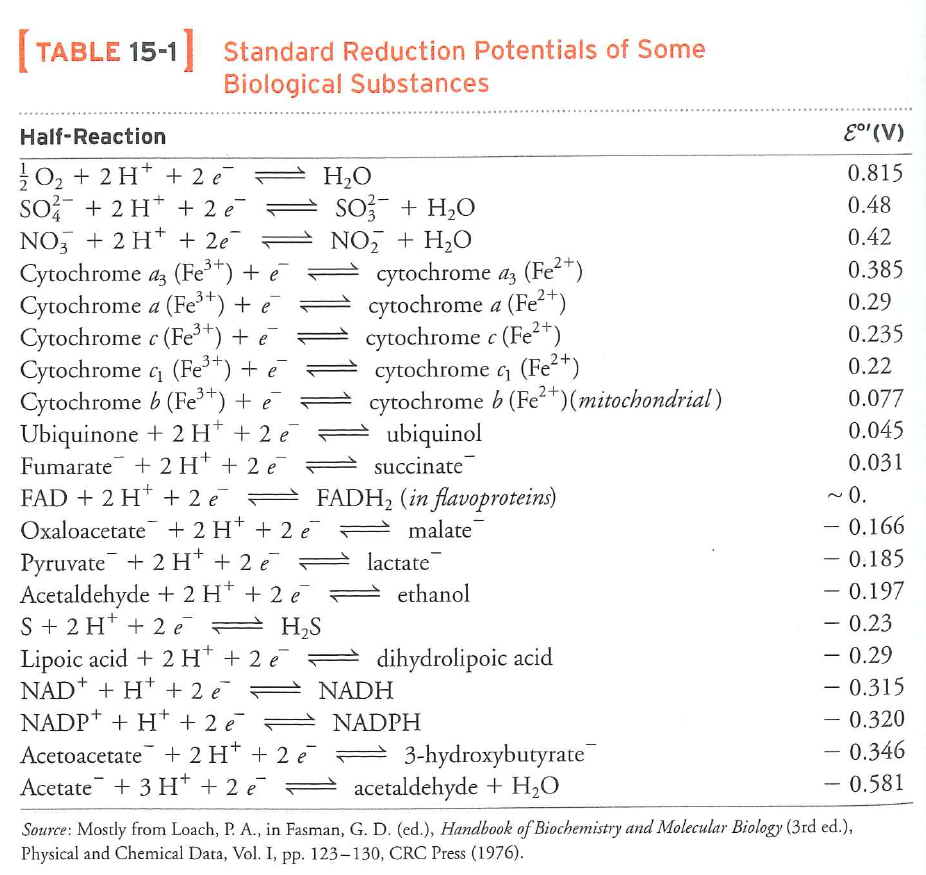
Finally: REDOX potentials measure electron affinity
Tables show reduction reactions
MORE POSITIVE MEANS HIGHER AFFINITY
Summary:
- Organisms extract energy from environment by oxidizing molecules
- They break this process up into many steps
- They control REDOX using $NADH$ (an electron carrier)
- They capture energy using $ATP$

