Glycolysis
2017-11-08
Conceptual goals
- Understand the chemical transformations by which glucose is converted to pyruvate
- Understand how the oxidation of glucose can release energy that can be captured as ATP
Skill goals
- Reason about metabolic energy extraction
- Reason about metabolic pathway regulation
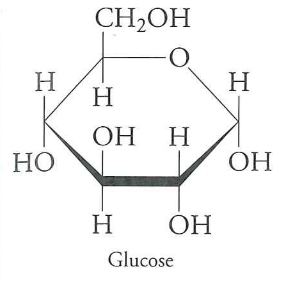
Are the carbons more or less oxidized than $CO_{2}$? Less
Than hexane? More
If we oxidize glucose to $CO_{2}$, we get -2850 kJ/mol
$C_{6}H_{12}O_{6} + 6O_{2} \rightarrow 6CO_{2} + 6H_{2}O$ would release all $2850\ kJ \cdot mol^{-1}$ at once.
This needs to be captured in incremental steps
Organisms have complex pathways for dealing with sugar "metabolites"
Glycolysis is a partial oxidation of glucose to two pyruvate molecules

Glycolysis is universal and ancient.
Glycolysis occurs in 10 steps, only three of which are highly favorable
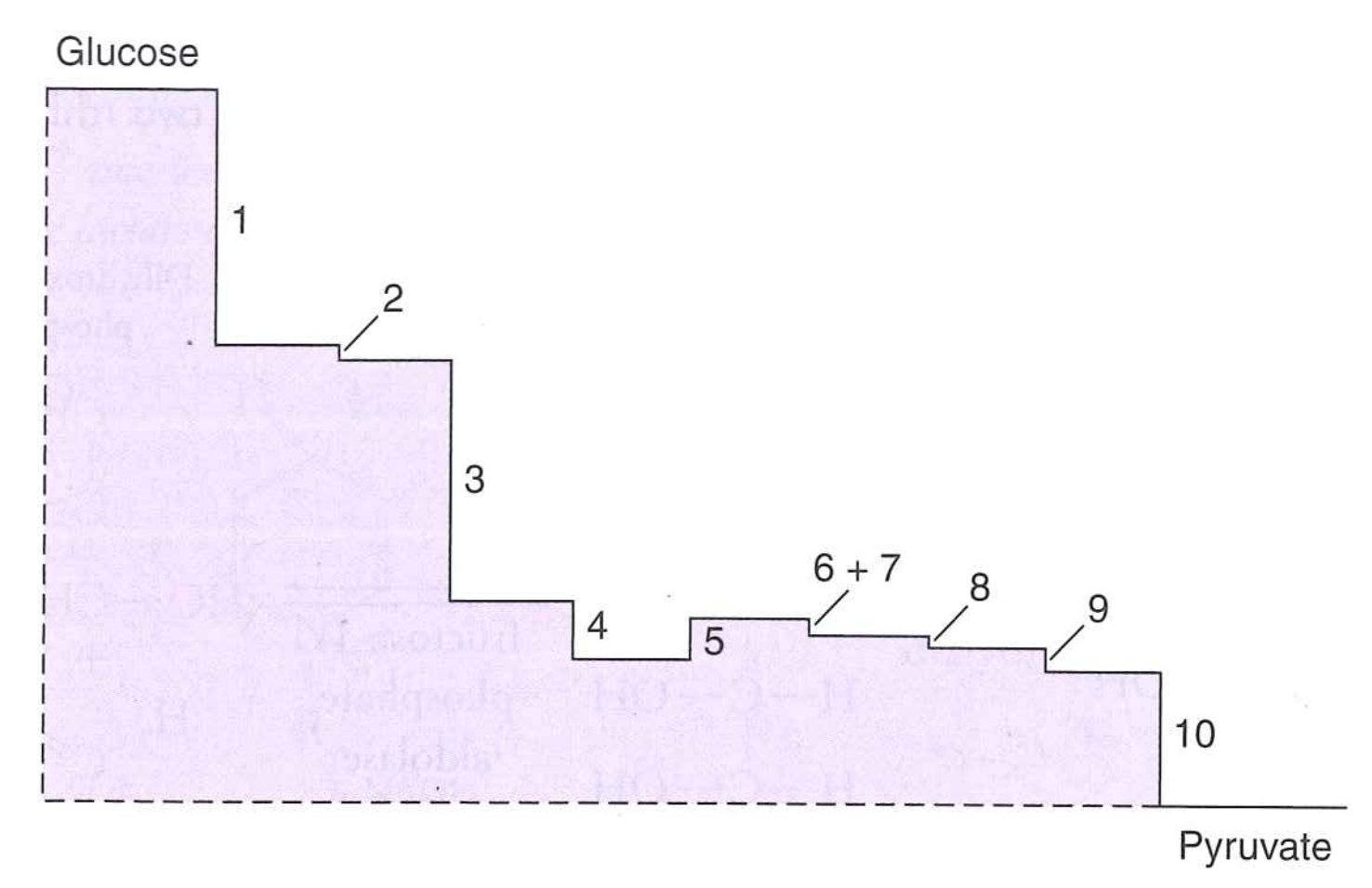
Glycolysis outline
- Pay 2 ATP
- Oxidize carbon and extract 4 ATP
glucose to glucose-6-phosphate
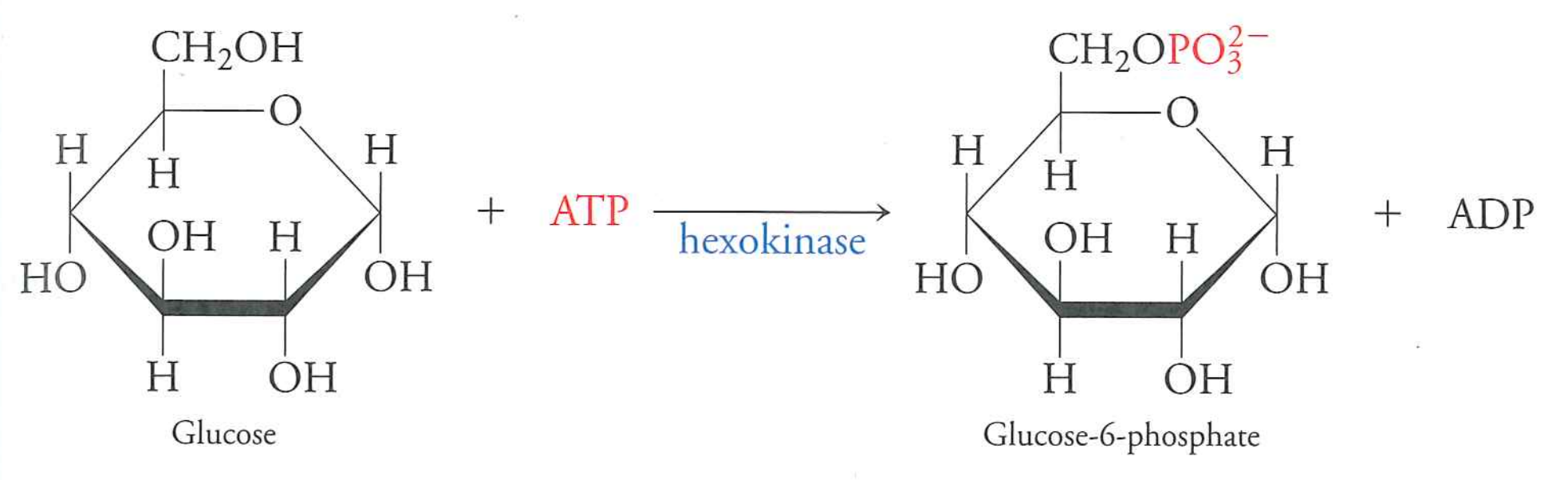
Uses hexokinase, burns one ATP
This is effectively irreversible (-16.7 kJ/mol).
Charge on G6P prevents diffusion out of cells
isomerize to fructose-6-phosphate
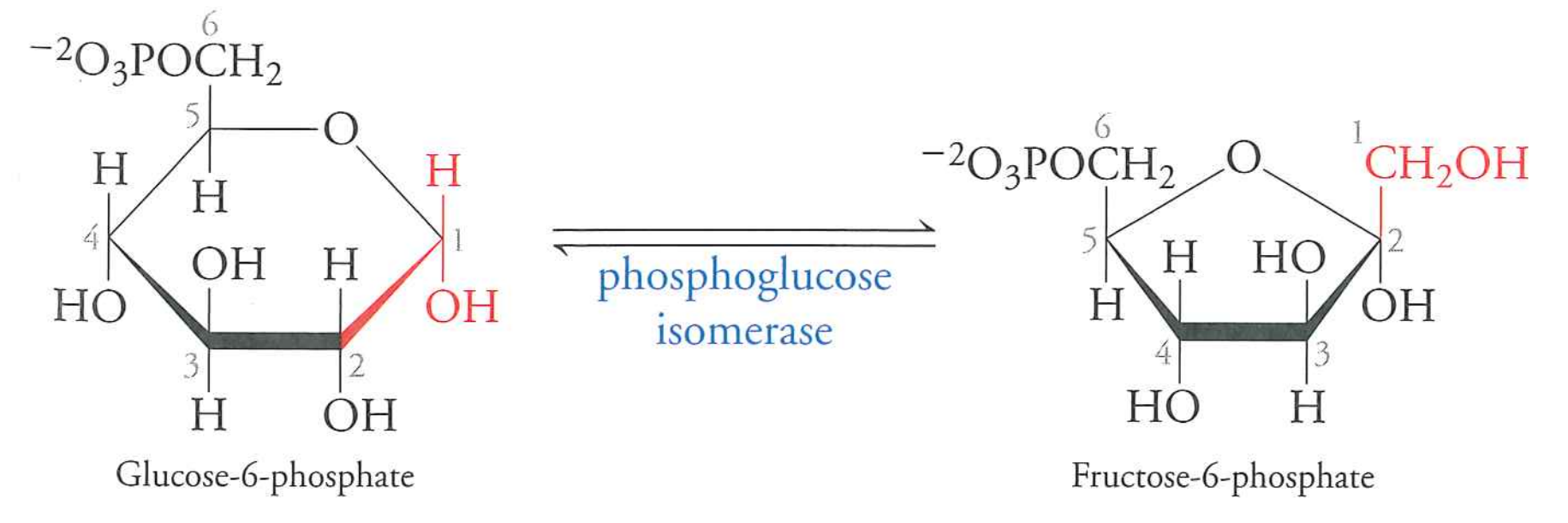
phosphoglucose isomerase
$\Delta G$ is -1.4 kJ/mol. Basically reversible.
General principle: intermediate steps often freely reversible.
fructose-6-phosphate to fructose-1,6-bisphosphate
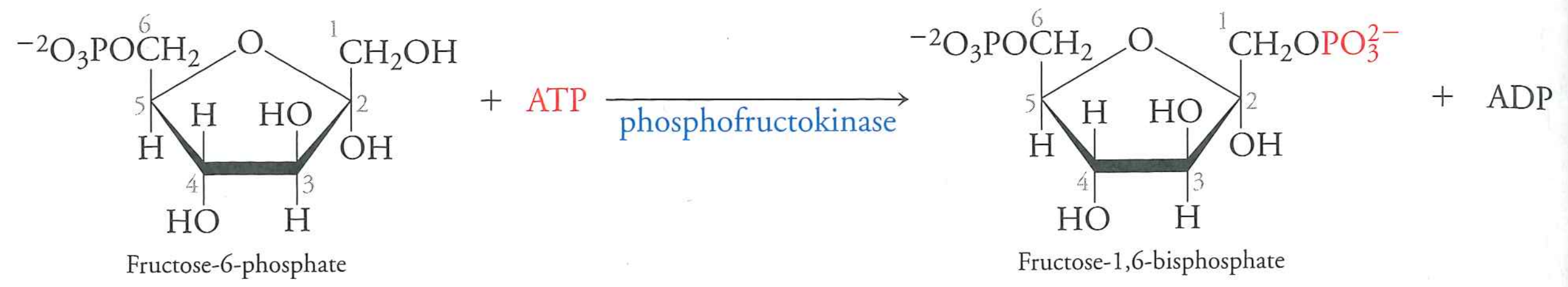
phosphofructokinase, burns one ATP
$\Delta G$ is -16.2 kJ/mol. This is an irreversible step.
This enzyme is allosterically regulated by ADP and PEP (bacteria) and fructose-2,6-biosphosphate (mammals)
Glycolysis is regulated at the phosphofructokinase step.
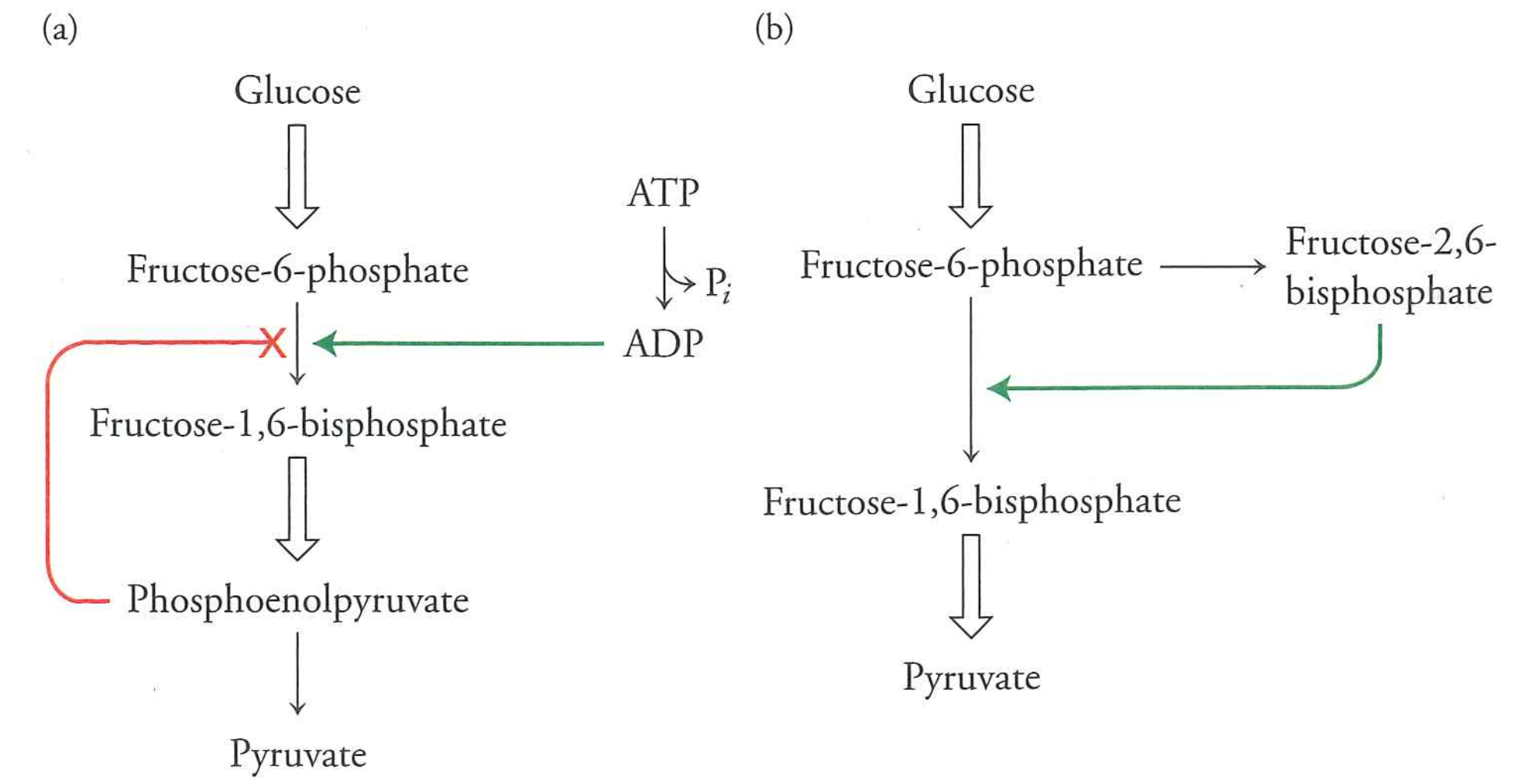
- Activated by excess ADP (bacteria) or excess glucose via fructose-1,6-bisphosphate (mammals)
- Deactivated by excess product (bacteria). "Product inhibition" is critical for many pathways
Why does it make sense to regulate a step with a large drop in $\Delta G$?


This makes a "one-way" valve.
fructose-1,6-bisphosphate to dihydorxyacetone phosphate and glyceraldehyde-3-phosphate
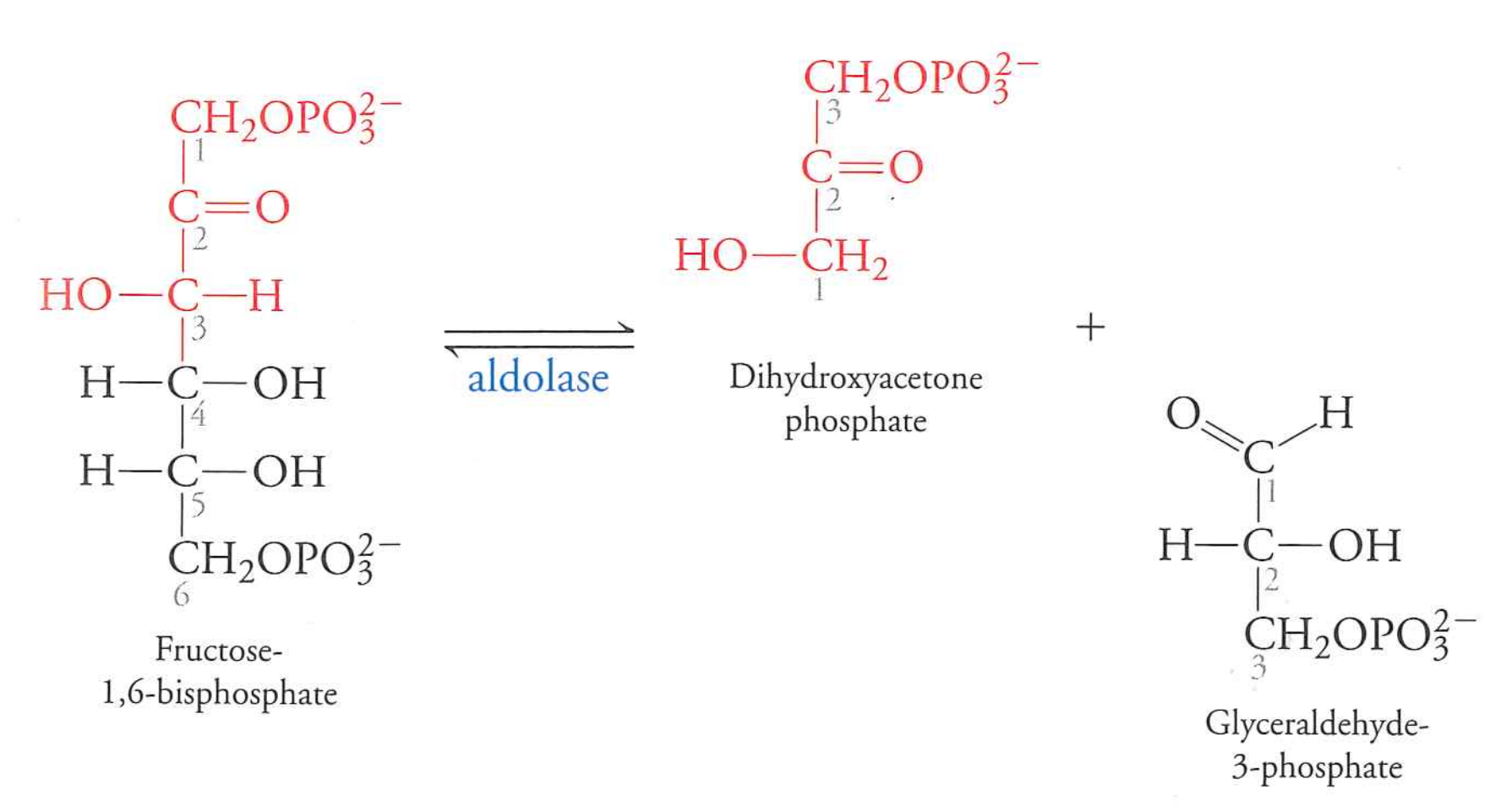
aldolase
$\Delta G$ near 0 in vivo
We've split glucose in half at this point.
DHAP gets converted to G3P
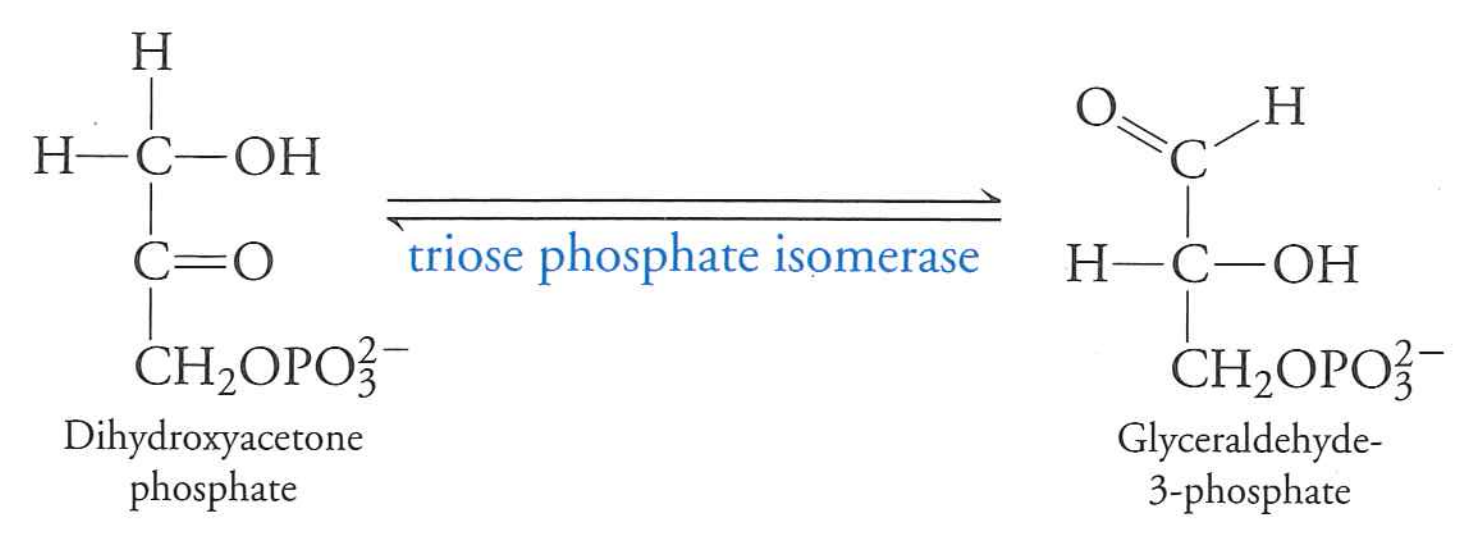
triose phosphate isomerase
$\Delta G$ slightly positive, but G3P gets used quickly thus "pulling" reaction forward.
Now we have two copies of glyceraldehyde-3-phosphate. The remaining pathway is doubled
Big ideas (so far):
- Metabolic pathways are usually a mixture of downhill irreversible steps and isoenergetic steps.
- Small unfavorable steps can be overcome by downstream favorable steps that "pull" material through the pathway.
- Irreversible steps are points of regulation because they can act as a one-way valve.
- Excess substrates often activate pathway (ADP or F26BP, indicating excess glucose).
- Products often inhibit a pathway via "product inhibition."
So far we've stuck two phosphates onto a sugar, rearranged it a few times, and broke it in half.
Discuss:
- Have we changed the oxidation state of any of the carbons? No.
- If we pulled the phosphates off could we recharge an ADP molecule? No.
We need to do some oxidation!
If we're going to oxidize carbon, we need to reduce something else. What will it be?
$NAD^{+}$
glyceraldehyde-3-phosphate + $NAD^{+}$ + $P_{i}$ $\rightarrow$ 1,3-bisphosphoglycerate + $NADH$ + $H^{+}$
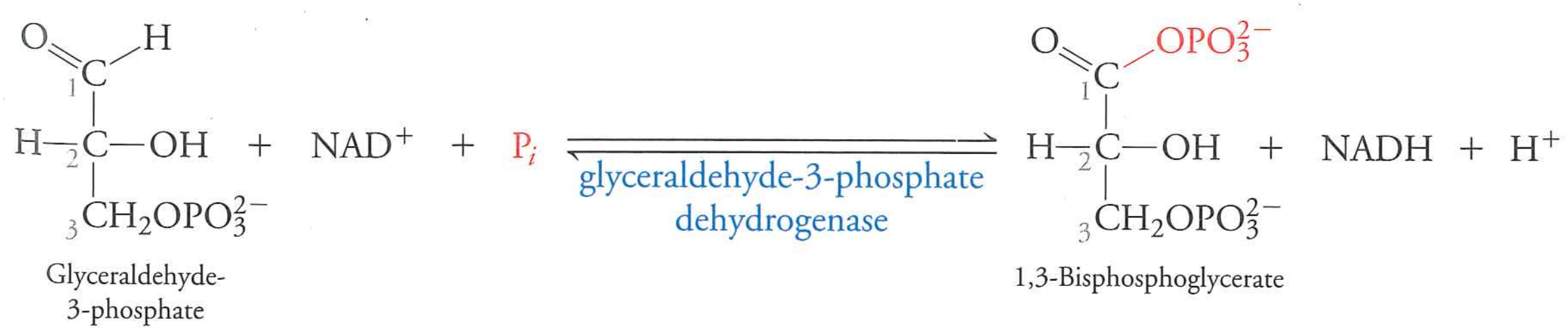
glyceraldehyde-3-phosphate dehydrogenase
oxidizes an aldehyde and extracts a hydride to $NAD^{+}$ to make $NADH$
unfavorable (+6.7 kJ/mol)
1,3 bisphosphoglycerate + ADP $\rightarrow$ 3-phosphoglycerate + ATP

phosphoglycerate kinase
$\Delta G$ is -18.8 kJ/mol, which lets this drag previous reaction forward
This is the first energy release. 1 ATP per 3-phosphoglycerate; 2 per glucose
3-phosphoglycerate $\rightarrow$ 2-phosphoglycerate

phosphoglycerate mutase
$\Delta G$ near 0
rearrangement of phosphate
2-phosphoglycerate $\rightarrow$ phosphoenolpyruvate
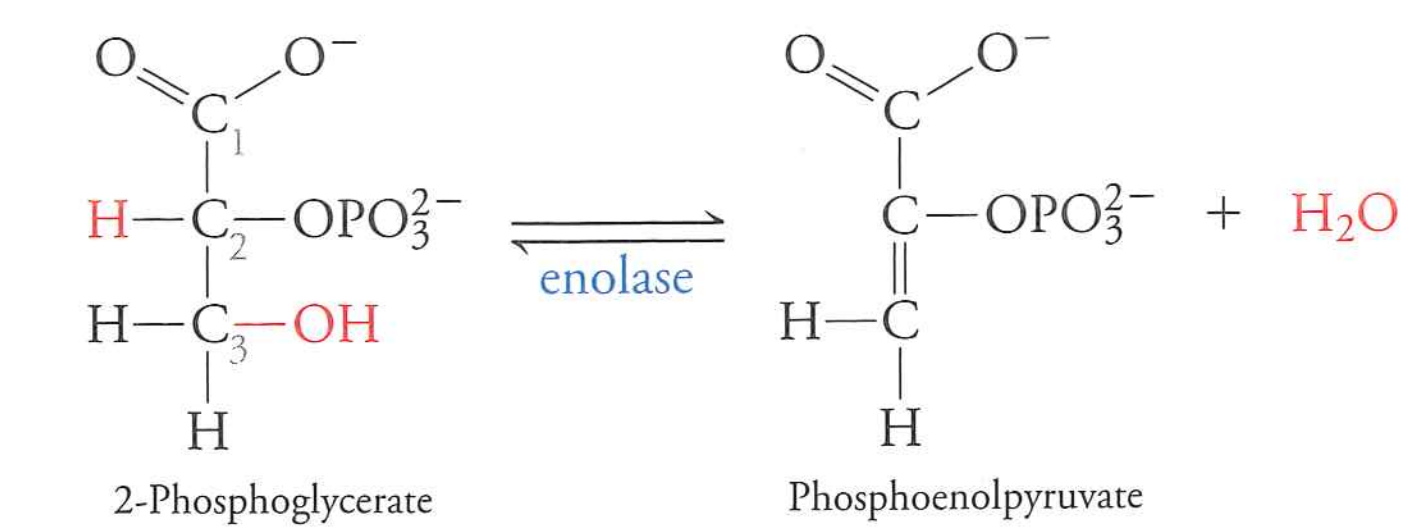
enolase
$\Delta G$ near 0
basic dehydration reaction
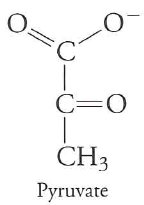
phosphoenolpyruvate $\rightarrow$ pyruvate

pyruvate kinase
yields 1 ATP per pyruvate (2 per glucose)
highly favorable, basically irreversible
Last step is three reactions that, together, are favorable
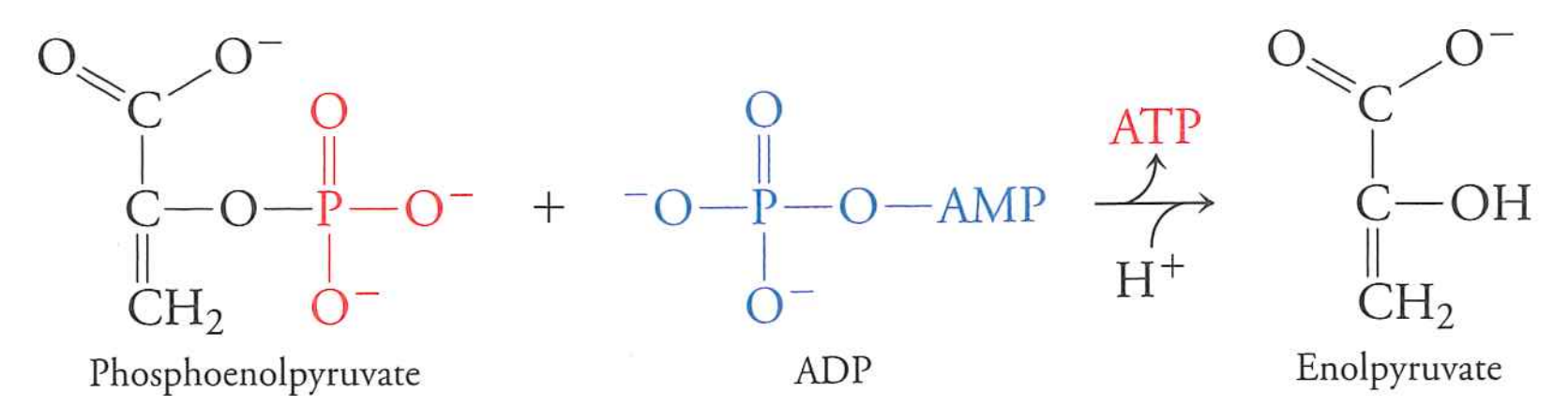
$PEP \rightarrow enolpyruvate$ (-16 kJ/mol) and $ADP + P_{i} \rightarrow ATP$ (30.5 kJ/mol)
Not enough to pay for making ATP.
Unfavorable reaction is coupled (in the same enzyme) to a favorable reaction
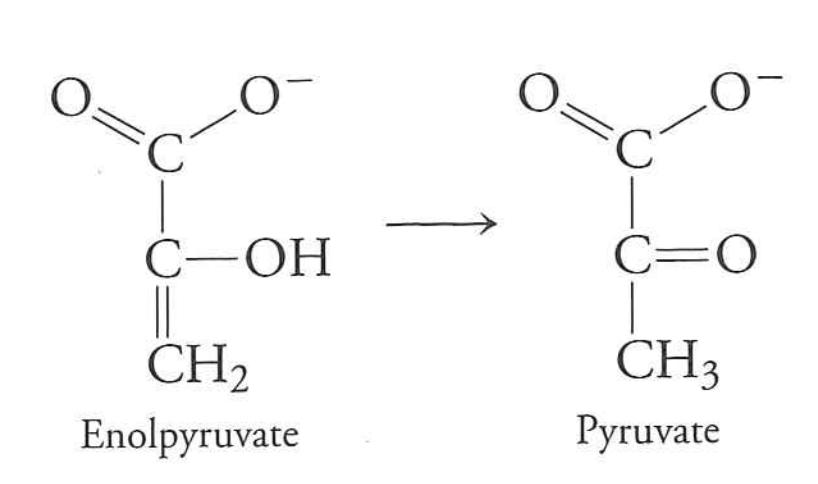
$enolpyruvate \rightarrow pyruvate$ is (-41 kJ/mol)
Reaction is net favorable by $-16 + 30.5 - 41 = -26.5\ kJ \cdot mol^{-1}$
Summary
- Each step is catalyzed by a distinct enzyme.
- Free energy consumed/released is transferred by ATP and NADH.
- Rate of the pathway can be controlled by altering activity of individual enzymes.
- Key, irreversible steps are regulated.
- Molecules like low-energy state (ADP) increase "flux"; high-energy state molecules (ATP) decrease "flux."
- Oxidation of carbon provides the energy to produce ATP.