Fermentation and Gluconeogenesis
2017-11-10
Conceptual goals
- Understand the chemical transformations by which $NADH$ is recycled
- Understand the chemical transformations by which pyruvate is converted to glucose
- Understand basic principles of metabolic energy extraction and regulation
Skill goals
- Reason about metabolic energy extraction
- Reason about metabolic pathway regulation
Organisms have complex pathways for dealing with sugar "metabolites"
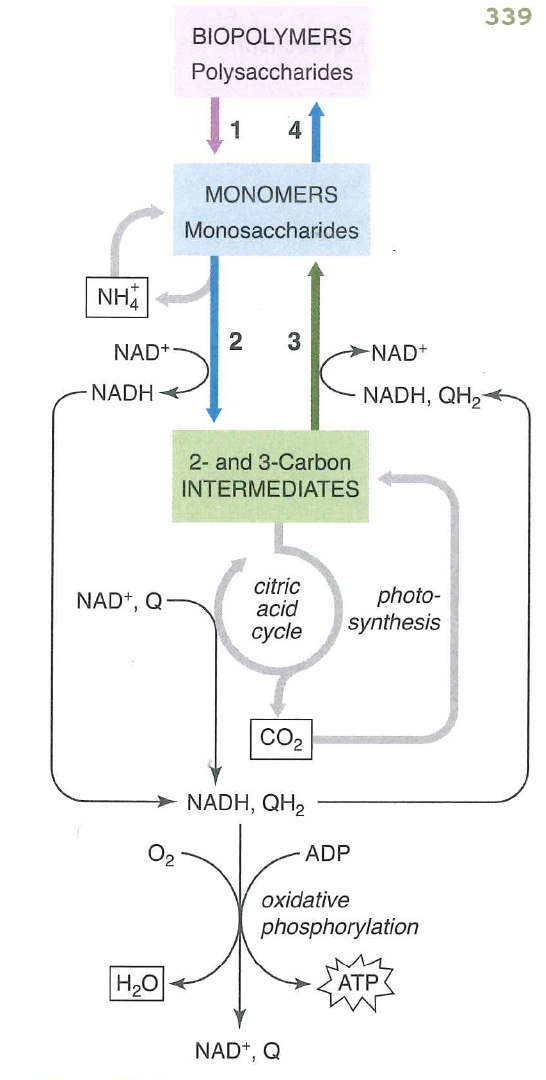
Metabolite is one of the small molecules passed around by cells in these complex pathways.
Quick review:
- What does the reaction-free-energy diagram look like for glycolysis? mixture of irreversible downhill steps and many "flat" steps"
- How does the whole process of glycolysis overcome small, unfavorable steps mid pathway? Favorable later steps "pull" material through
Quick review:
- Why are steps with large drop in $\Delta G$ good points of regulation? Create one way "valves" to stop "flux" through the pathway.
- What is product inhibition, and how does it allow a cell to control "metabolite" concentrations? The product of the pathway inhibits an early enzyme in the pathway, meaning the cell "knows" when it has made enough product and stops the pathway
Outline
REDOX!
What happens if a cell runs out of $NAD^{+}$? Fermentation.
What happens if an organism needs more glucose? Gluconeogenesis.
Part-the-zeroth: REDOX
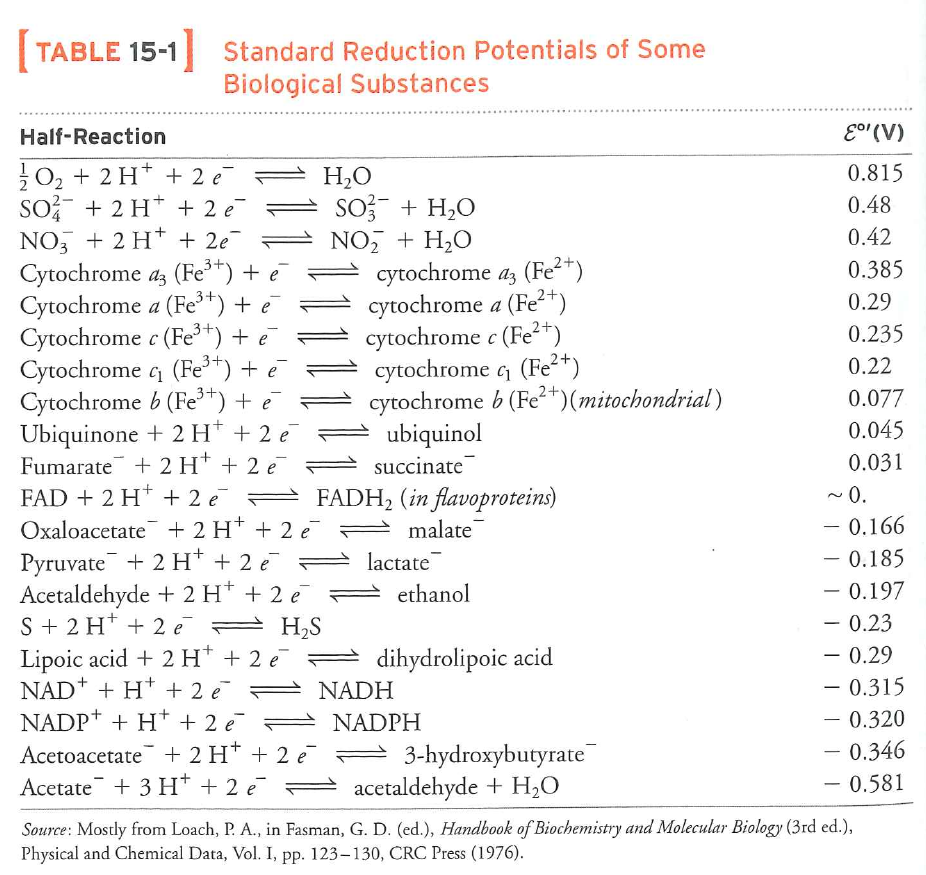
Rules
- Moving up the table is favorable
- To figure out how favorable, determine $\Delta \varepsilon$
- To figure out how much energy is released in a reaction, calculate $\Delta G = -n F \Delta \varepsilon$ (where $n$ is number of electrons and $F$ is $96 \ kJ \cdot V^{-1}$)
What is the energy to oxidize $NADH$ using $O_{2}$?
$-217\ kJ \cdot mol^{-1}$
Part-the-first: fermentation
What if we run out of $NAD^{+}$?
You can't oxidize glucose (or many other metabolites) and you die.
What kind of reaction would we need to drive $NADH \rightarrow NAD^{+} + H^{-}$?
Reduction, to oxidize $NADH$

Any above $NADH$ on this table
Most creatures: pyruvate to lactate via lactic acid fermentation
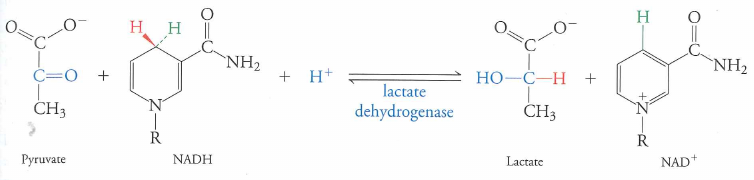
End result is recycled $NAD^{+}$.
Excess lactate + excess $NAD^{+}$ will run this in reverse
Practice: what is the energy change for lactate fermentation?
$\Delta G ^{\circ \prime} = -n F \Delta \varepsilon ^{\circ \prime}$
$NAD^{+} + H^{-} \rightarrow NADH$, $\varepsilon ^{\circ \prime} = -0.315\ V$
$pyruvate\ + H^{-} \rightarrow lactate$, $\varepsilon ^{\circ \prime} = -0.185\ V$
Step 1: Calculate the change in the redox potential (the change in electron affinity)
$\Delta \varepsilon^{\circ \prime} = \varepsilon_{acceptor} - \varepsilon_{donor}$
$\Delta \varepsilon^{\circ \prime} = \varepsilon_{pyr \rightarrow lac} - \varepsilon_{nad+ \rightarrow nadh}$
$\Delta \varepsilon^{\circ \prime} = (-0.185\ V-- 0.315\ V)$
$\Delta \varepsilon^{\circ \prime} = 0.130 \ V$
Step 2: Calculate the change in the energy
$\Delta \varepsilon^{\circ \prime} = 0.130 \ V$
$\Delta G ^{\circ \prime} = -n F \Delta \varepsilon ^{\circ \prime}$
$\Delta G ^{\circ \prime} = -2 \times 96\ kJ\cdot V^{-1} mol^{-1} 0.130 \ V $
$\Delta G ^{\circ \prime} = -25 \ kJ \cdot mol^{-1}$
Not quite enough for an ATP
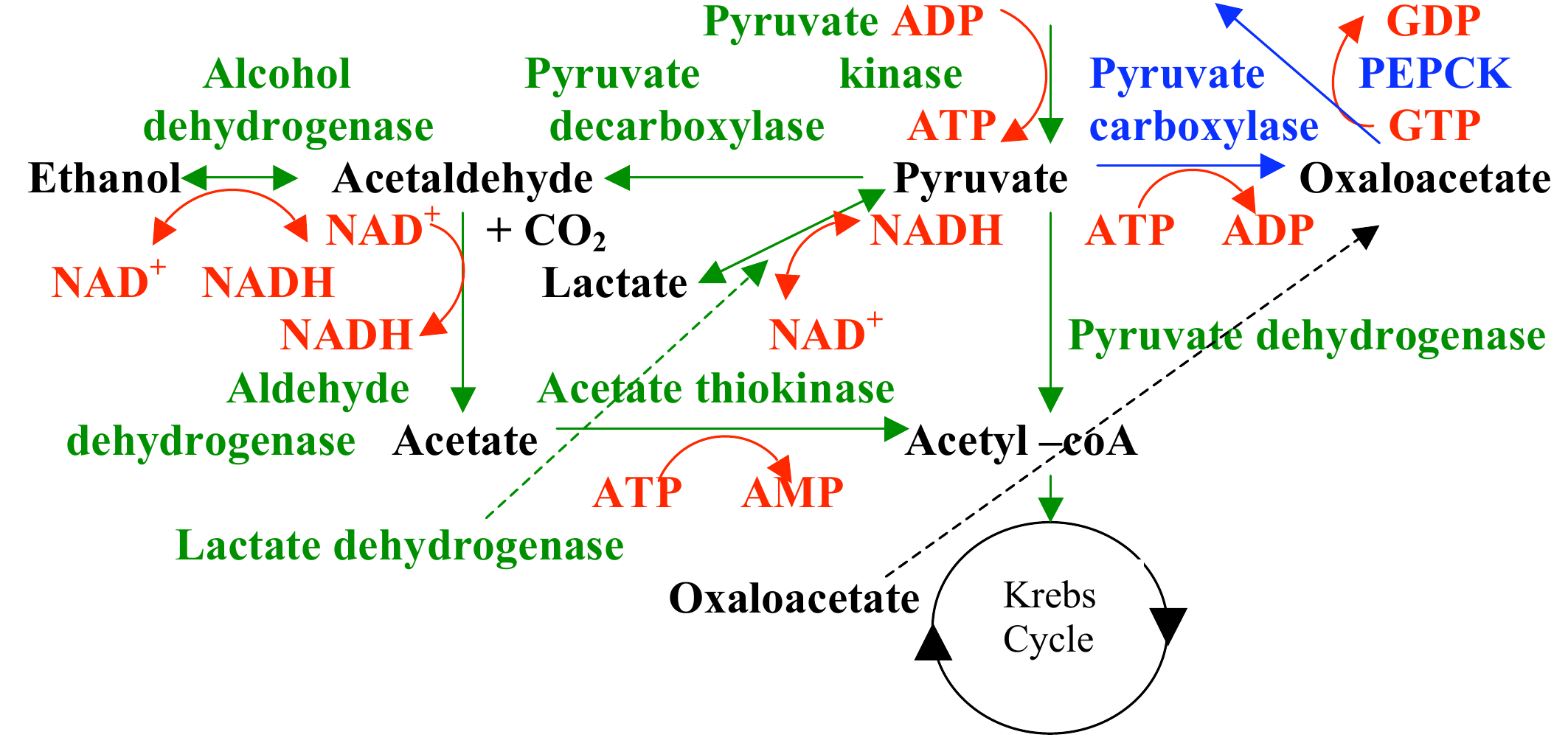
What do yeast do to recycle their $NADH$?
$pyruvate \rightarrow acetaldehyde \rightarrow EtOH$
$acetaldehyde \rightarrow EtOH$ is a reduction that oxidizes $NADH$ to $NAD^{+}$
Key points:
- $NADH$ must be recycled to $NAD^{+}$ (oxidized) for glycolysis to continue
- This occurs by fermentation (either lactate or ethanol)
- $NADH$ oxidation itself releases energy, but we need a higher-affinity acceptor to actually capture $ATP$

Mini homework question:
How many $ADP + P_{i} \rightarrow ATP$ reactions could we pay for if we took the electrons from $NADH$ and stuck them on $O_{2}$?
Part-the-second: gluconeogenesis
Human brain runs (almost) solely on glucose.
If you don't eat glucose, where can you get some?
Gluconeogenesis:
$2\ pyruvate + 4ATP + 2GTP + 2NADH + 2H^{+} + 4H_{2}O \rightarrow $
$glucose + 4ADP + 2GDP + 6P_{i} + 2NAD^{+}$
Glycolysis:
$glucose + 2ADP + 2P_{i} + 2NAD^{+} \rightarrow $
$2\ pyruvate + 2NADH + 2ATP + 2H^{+} + 2H_{2}O$
What is the difference in the energy released by glycolysis versus taken up by gluconeogenesis?
$glucose + 2ADP + 2P_{i} + 2NAD^{+} \rightarrow $ $2\ pyruvate + 2NADH + 2ATP + 2H^{+} + 2H_{2}O$vs.
$2\ pyruvate + 4ATP + 2GTP + 2NADH + 2H^{+} + 4H_{2}O \rightarrow $ $glucose + 4ADP + 2GDP + 6P_{i} + 2NAD^{+}$$ATP \rightarrow ADP + P_{i} = -30.5\ kJ \cdot mol^{-1}$
$NADH=3ATP$
-244 vs. +366 kJ/mol
Why does it cost more to go backwards than forwards?
Entropy.
Big idea:
Catabolic pathways (breaking things down) generally release energy
Anabolic pathways (building things up) generally consume energy
Can gluconeogenesis just use the same enzymes as glycolysis, run in reverse?
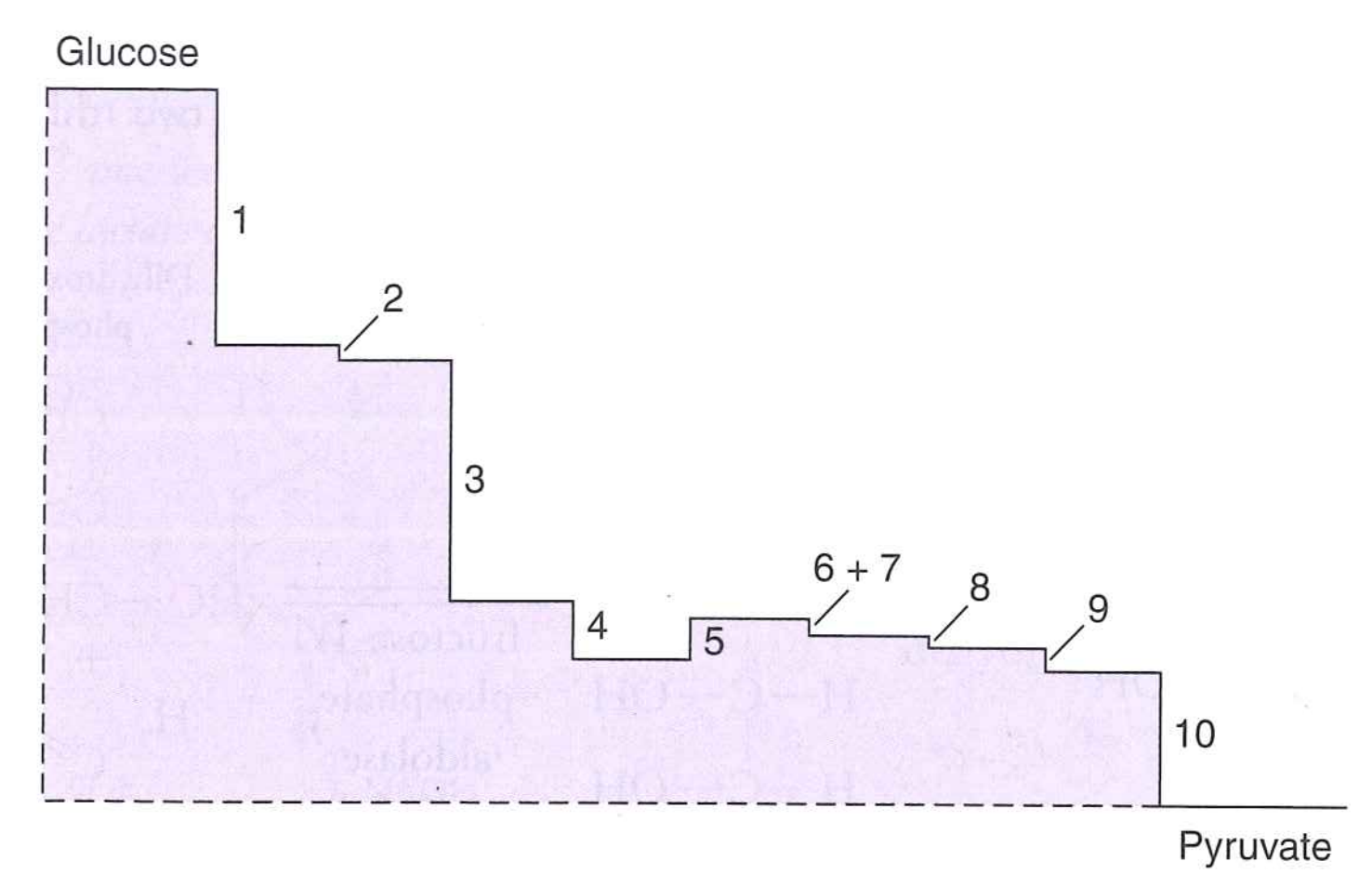
No. There are irreversible steps!
Gluconeogenesis uses three bypass reactions to reverse glycolysis.
$pyruvate + ATP \rightarrow PEP + ADP$; $\Delta G = +16.7\ kJ\cdot mol^{-1}$
$F1,6BP + ADP \rightarrow F6P + ATP$; $\Delta G = +22.2\ kJ\cdot mol^{-1}$
$G6P + ADP \rightarrow glucose + ATP$; $\Delta G = +33.4\ kJ\cdot mol^{-1}$
These guys are "easy": just lop off the phosphate.
$F1,6BP + ADP \rightarrow F6P + ATP$; $\Delta G = +22.2\ kJ\cdot mol^{-1}$
$F1,6BP \rightarrow F6P + P_{i}$; $\Delta G = -16.3\ kJ\cdot mol^{-1}$
$G6P + ADP \rightarrow glucose + ATP$; $\Delta G = +33.4\ kJ\cdot mol^{-1}$
$G6P \rightarrow glucose + P_{i}$; $\Delta G = -13.8\ kJ\cdot mol^{-1}$
What about pyruvate to PEP?
$pyruvate + ATP \rightarrow PEP + ADP$; $\Delta G = +16.7\ kJ\cdot mol^{-1}$
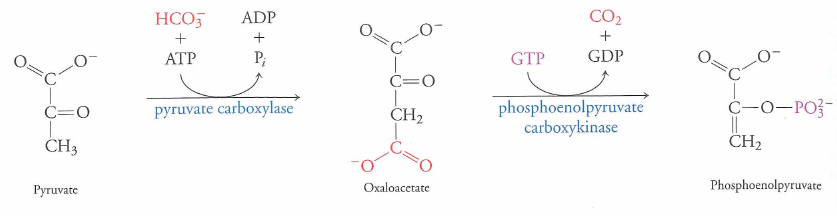
$\Delta G = 0.9\ kJ\cdot mol^{-1}$
What would happen if a cell ran glycolysis and gluconeogenesis at the same time?
Burn ATP and generate heat.
Glycolysis and gluconeogenesis are reciprocally regulated
The same molecule that turns on glycolysis turns off gluconeogenesis (and vice versa)
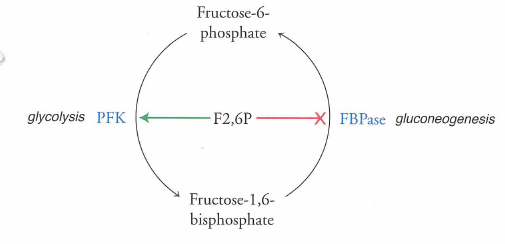
Big point: metabolic pathways can transform one "metabolite" into another in a regulated fashion.
Pathway summary
- Fermentation is used to oxidize $NADH$ so glycolysis can continue
- This is necessary if $NAD^{+}$ is depleted, but leaves energy "on the table"
- Gluconeogenesis allows synthesis of glucose from pyruvate
- This is energetically costly
- Requires "bypass" of irreversible steps in glycolysis
- Is "reciprocally-regulated" with glycolysis
Big idea summary
Catabolic pathways (breaking things down) generally release energy
Anabolic pathways (building things up) generally consume energy
Metabolic pathways can transform one "metabolite" into another in a regulated fashion.