Oxidative Phosphorylation II
2017-11-20
Conceptual goals
- Understand how the electron transport chain couples movement of electrons along a REDOX gradient to creating a proton gradient
- Understand how different cofactors can safely shuttle electrons
Skill goals
- Reason about the linkage between changes in reduction potential and ability to transport protons against a concentration gradient
- Reason about the relative ordering of the electron transport chain based on REDOX potentials and perturbation with drugs
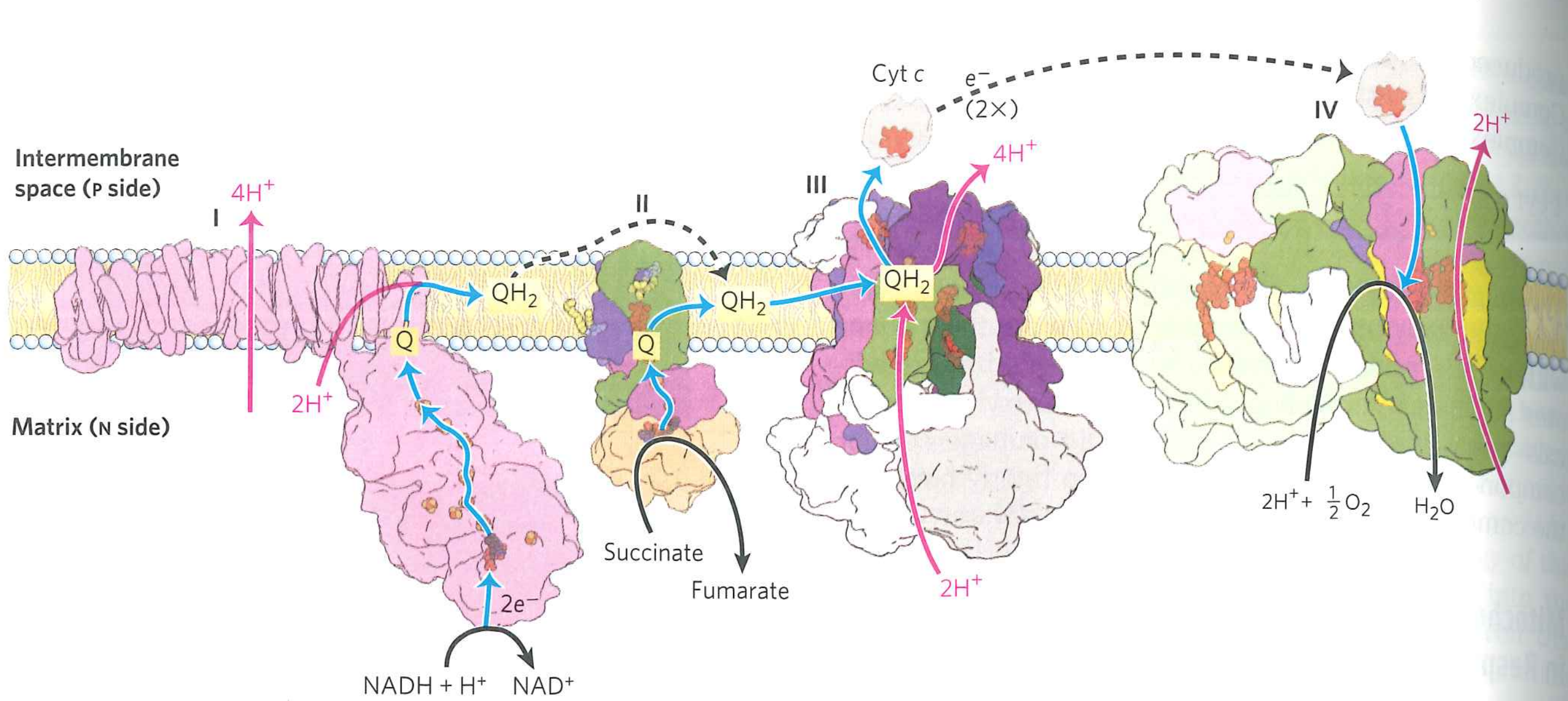
Oxidative phosphorylation overview:
Why does oxidative phosphorylation break the $NADH + \frac{1}{2}O_{2} + H^{+} \rightarrow NAD^{+} + H_{2}O$ reaction into multiple steps?
Control, capture of energy at each step
Where does ox-phos occur (and which way are protons pumped)?
In the mitochondria, pumping protons from matrix into intermembrane space
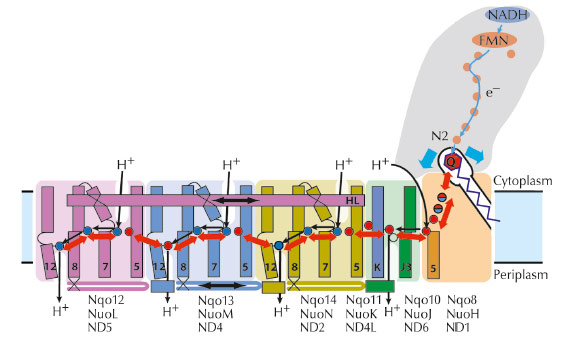
Complex I couples oxdiation of $Q$ to $QH_{2}$ to transport of protons across the bilayer
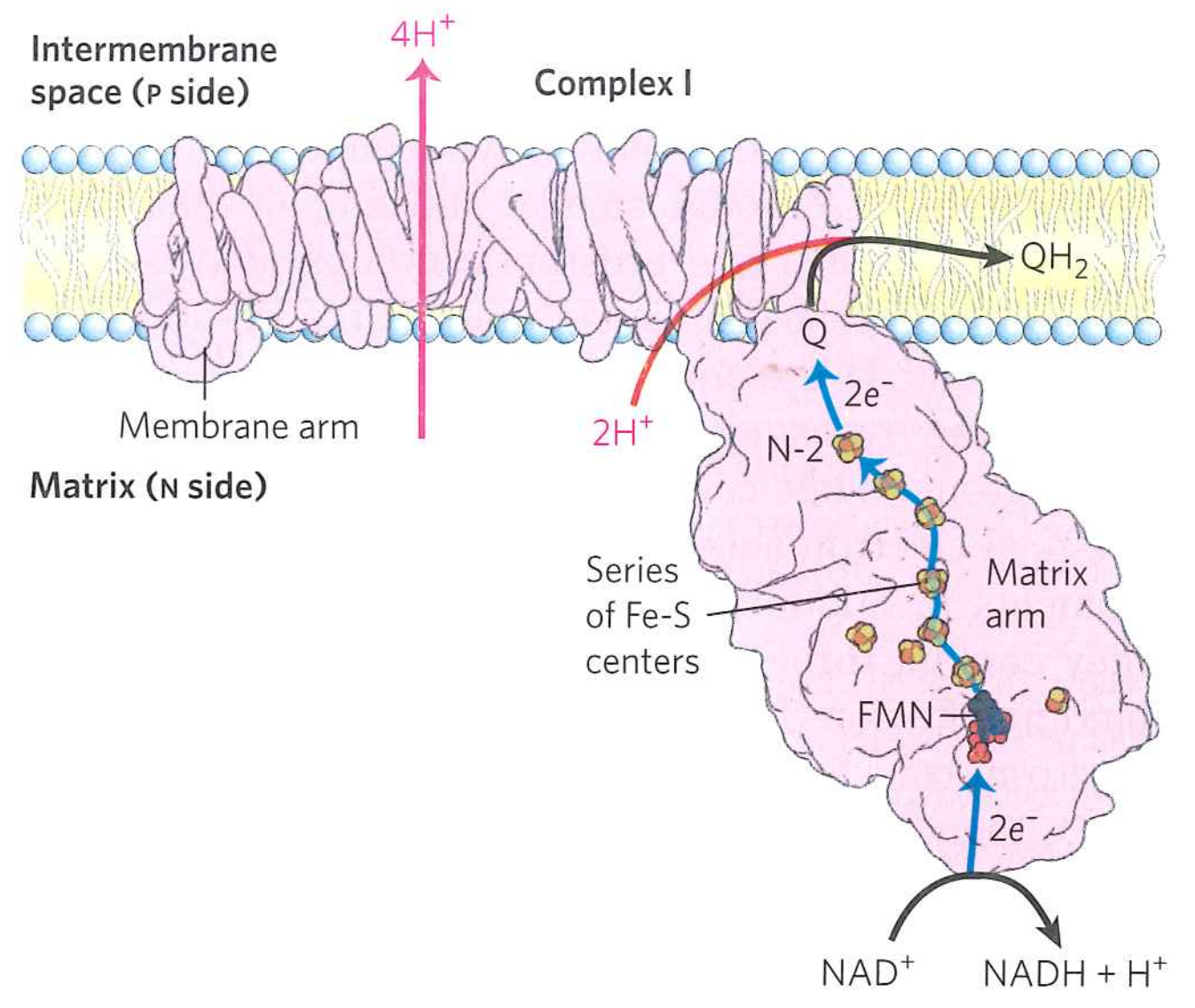
Process has many sequential steps
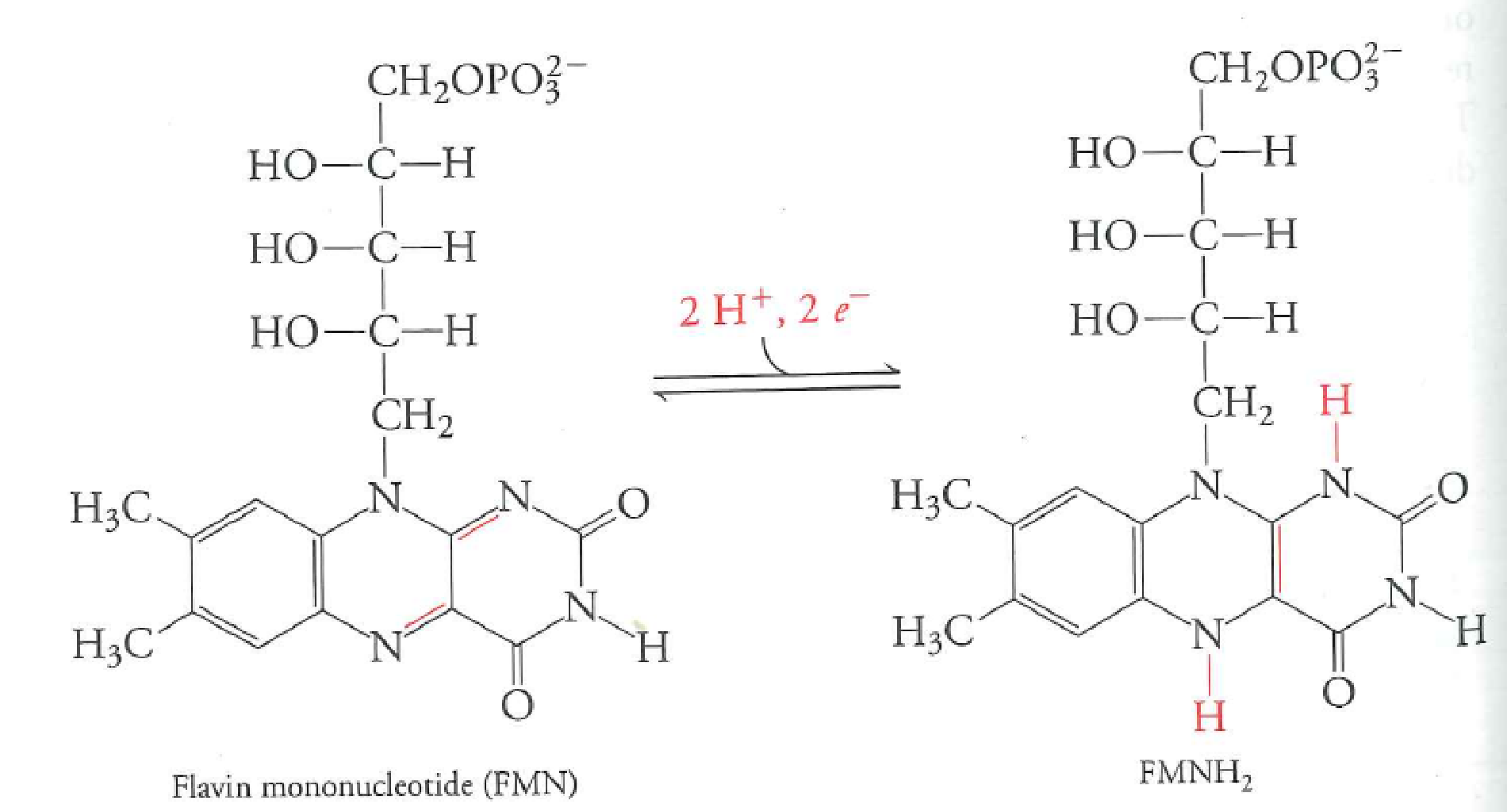
$NAD:H + H^{+} + FMN \rightarrow FMNH_{2}$
$FMNH_{2} + 2Fe^{3+} \rightarrow FMN + H_{2} + 2Fe^{2+}$
Pass along several iron-sulfur clusters
$2Fe^{3+} + 2Fe^{2+} \rightarrow 2Fe^{2+} + 2Fe^{3+}$
$2Fe^{2+} + Q + 2H^{+} \rightarrow 2Fe^{3+} QH_{2}$
FMN accepts electrons directly from NADH
Iron-sulfur clusters
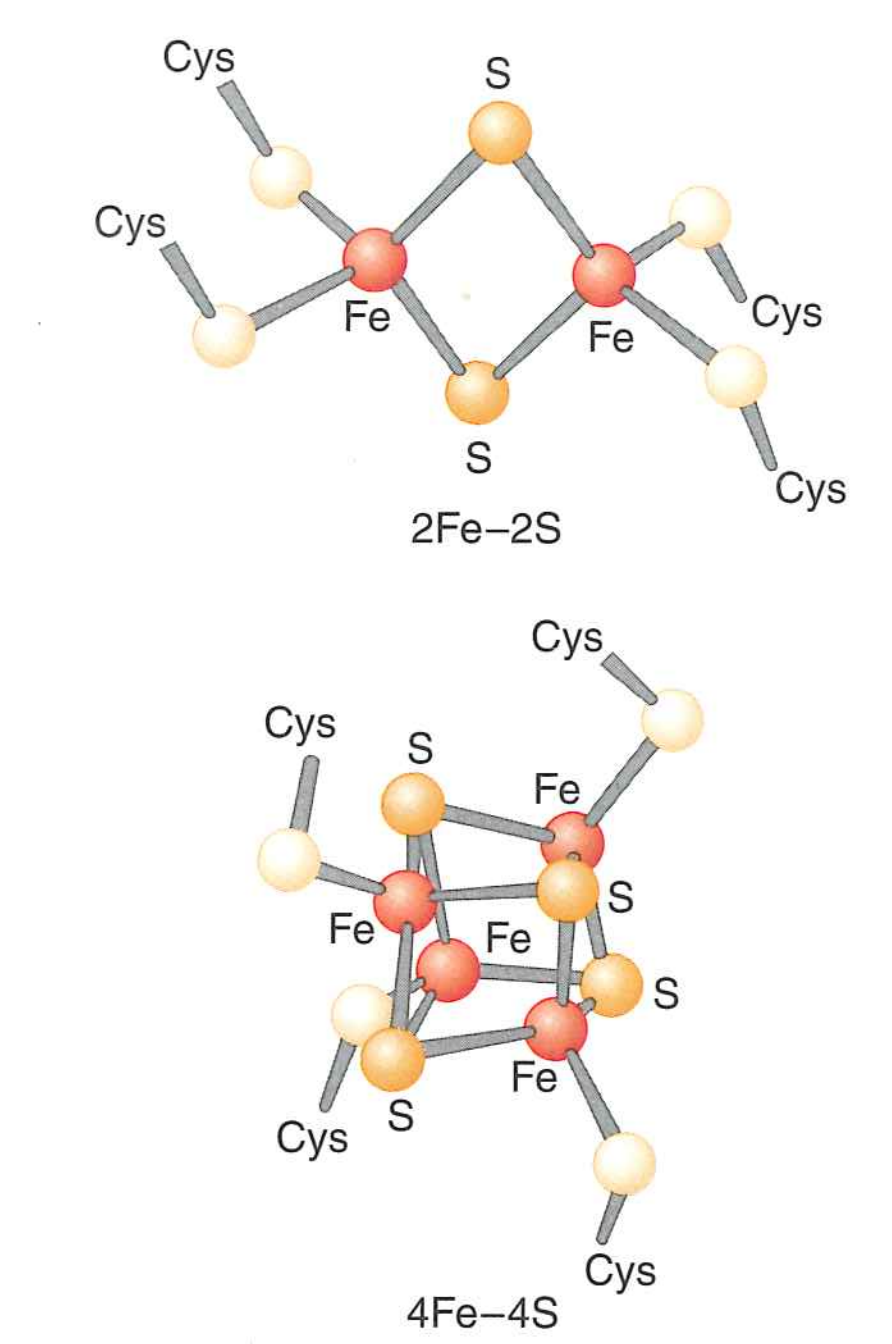
Iron-sulfur clusters
- Driven by oxidation of $Fe^{2+}$ to $Fe^{3+}$.
- Single electron transfer
- Sulfur complexes hold $Fe$ iron away from water and physically links oxidation state of $Fe$ to rest of protein
The mysterious $Q$ (ubiquinone, coenzyme Q10)
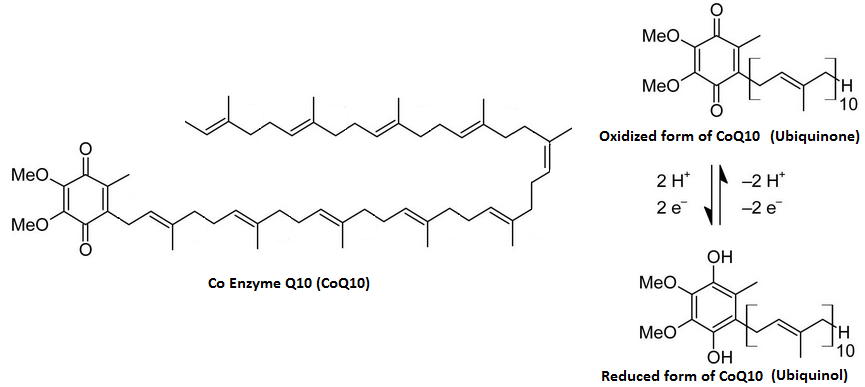
Source: examine.com
Ubiquinone
- Driven by oxidation carbonyl/hydroxyls on conjugated ring
- Two electron transfer
- Both oxidized and reduced forms are uncharged. Diffuses in the bilayer

Key point: the $2H^{+}$ come from the matrix side
Transfer is also linked to transfer of four more protons...somehow.
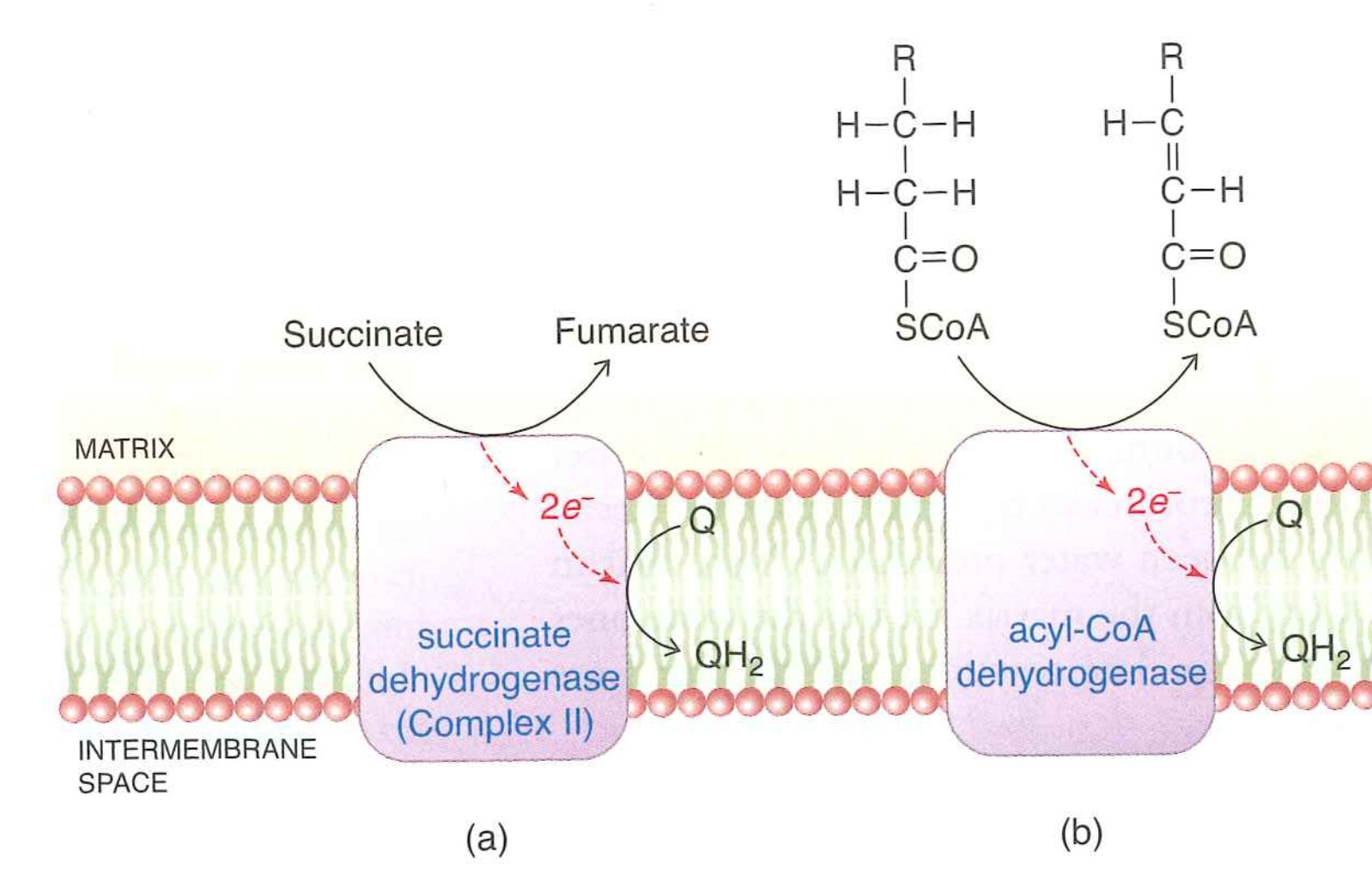
Complex I is not the only source of $QH_{2}$.
Succinate enters oxidative phosphorylation via Complex II, generating $QH_{2}$
Fatty acid oxidation, other oxidation reactions can generate $QH_{2}$ using a variety of enzymes
What happens to $QH_{2}$?

Enters "Q-cycle" which ultimately oxidizes cytochrome C and moves $4H^{+}$ across the bilayer
$2QH_{2} \rightarrow QH_{2} + Q + 2H^{+}$
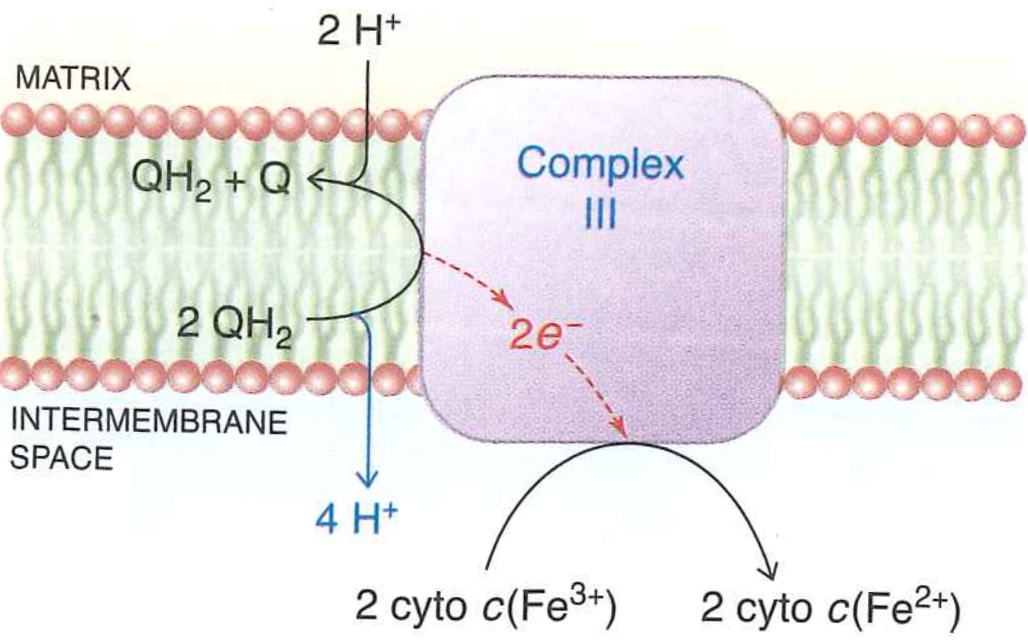
Key point: this is an indirect pump. Geometry of complex III means protons are preferentially picked up on matrix side, preferentially dropped on lumen side

Oxidative phosphorylation overview:

Cytochromes
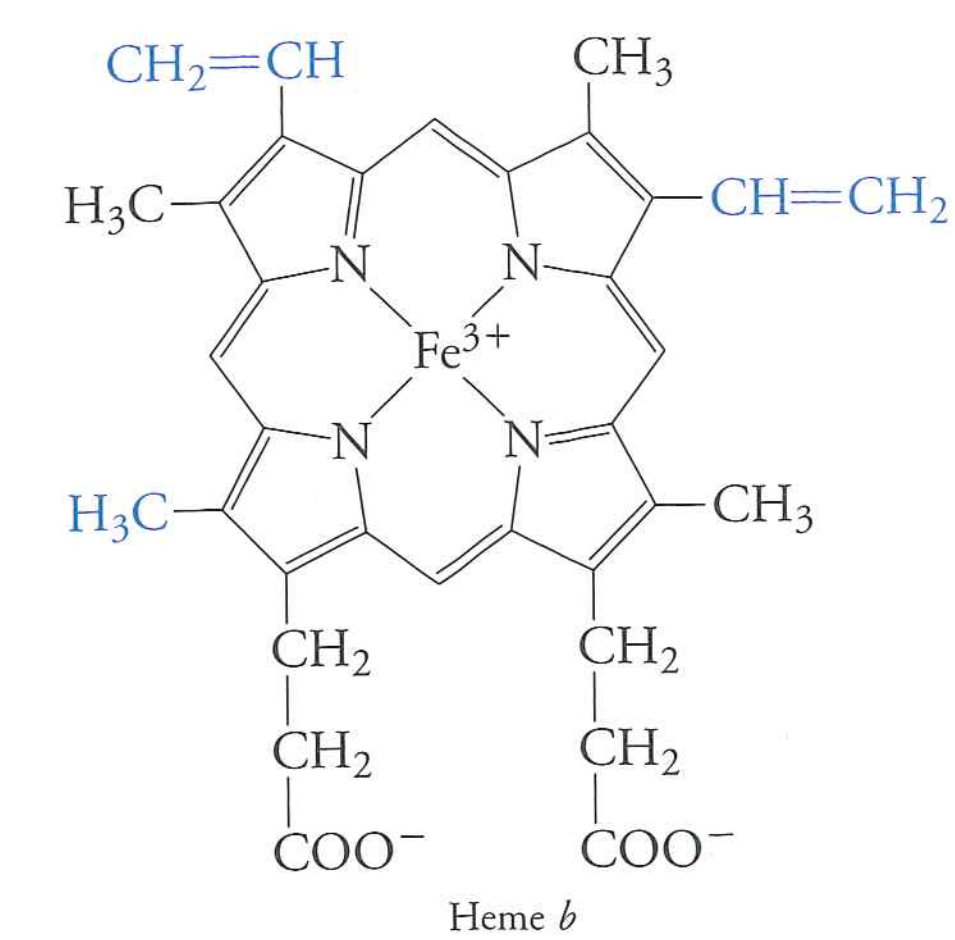
- Driven by oxidation of $Fe^{2+}$ to $Fe^{3+}$.
- Single electron transfer
- Heme holds $Fe$ iron away from water and physically links oxidation state of $Fe$ to rest of protein
Cytochromes hold onto this heme ring in a deep pocket, protecting Fe
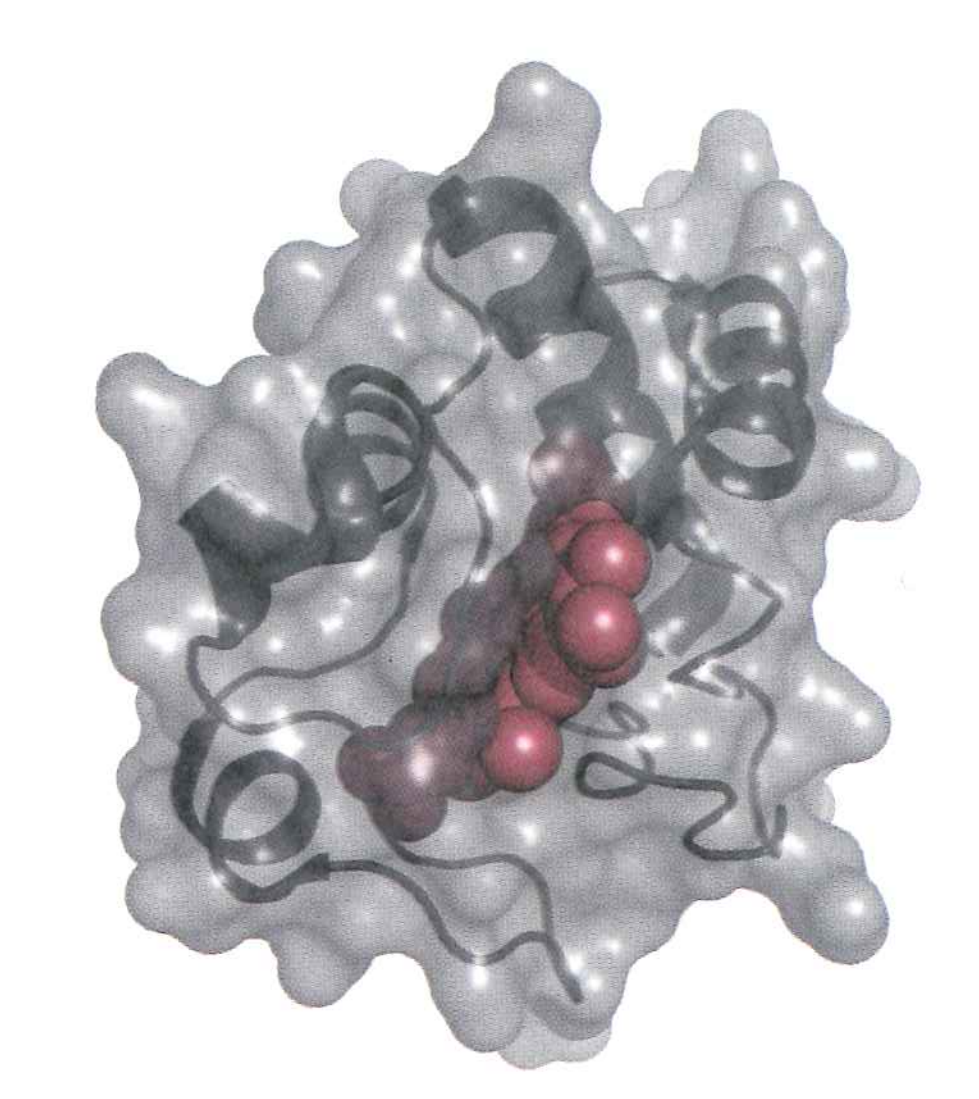
Cytochrome c is a "peripheral" membrane protein that "skates" over to complex IV
Oxidative phosphorylation overview:

Complex IV uses $O_{2}$ to oxidize $CytC(Fe^{2+})$.
$4\ CytC(Fe^{2+}) + O_{2} + 4H^{+} \rightarrow 4\ CytC(Fe^{3+}) + 2H_{2}O$
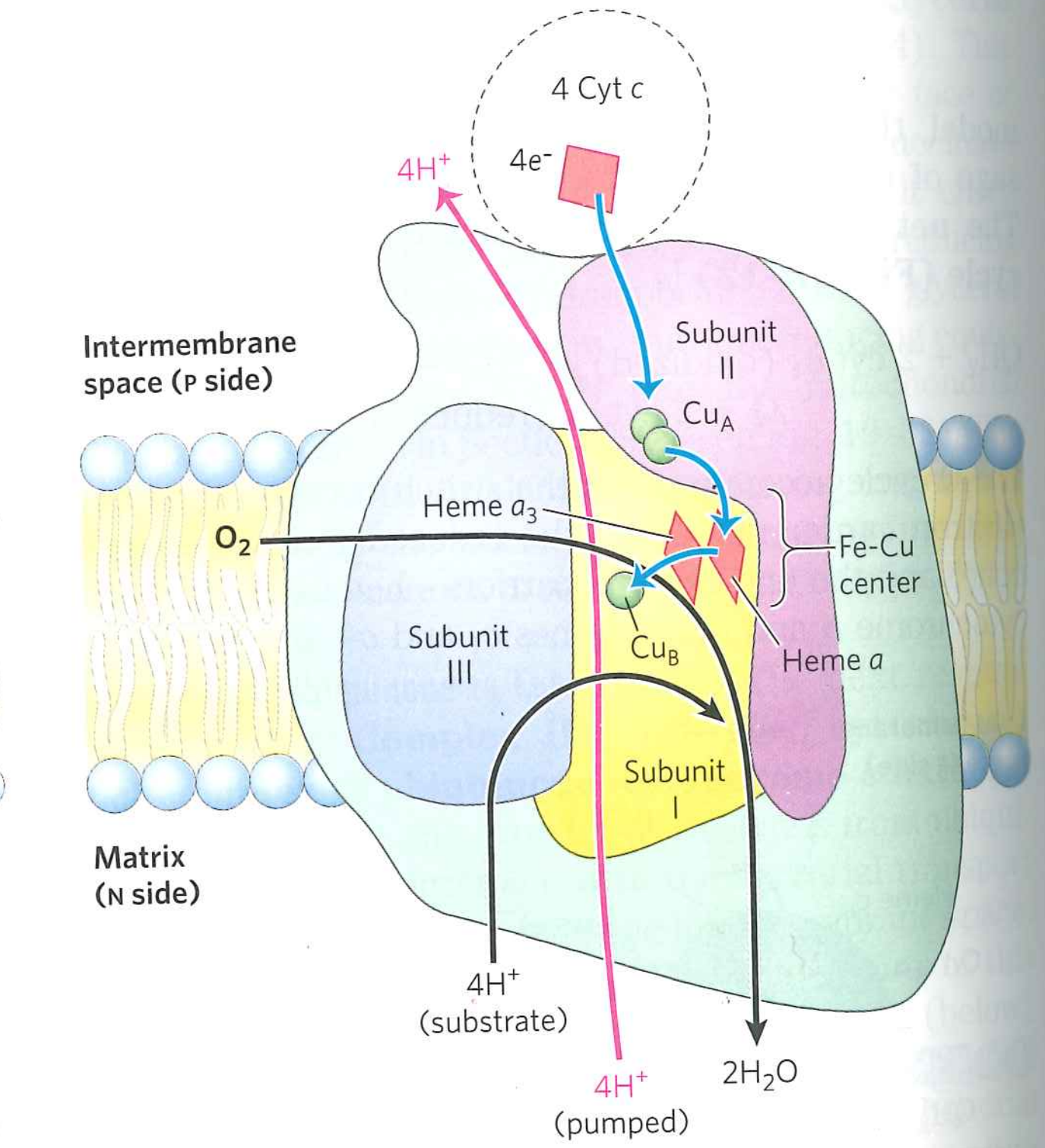
This transports 2 more protons across the bilayer

Why might succinate to fumarate come in to ox-phos directly as $QH_{2}$ (rather than going through complex I)?
Does this give as much energy as an NADH?
$succinate$ has higher affinity for $e^{-}$ than $NADH$
$fumarte + 2H^{+} + 2e^{-} \rightarrow succinate^{-}$, $\varepsilon^{\circ \prime} = 0.031 \ V$
$NAD^{+} + H^{-} \rightarrow NADH$, $\varepsilon^{\circ \prime} = -0.315 \ V$
4 fewer protons are transported for succinate than $NADH$: lower energy yield
Highest yield step?
$QH_{2} \rightarrow cytochrome\ C$
Surprising?
Why not transport more $H^{+}$ at the $O_{2}$ step? I'm not sure, actually
Why might electron transfers be so tightly controlled?
Premature contact with $O_{2}$ can generate highly reactive $\cdot O_{2}^{-}$ radical.
Summary I
- Passage through Complexes I-IV allows oxidation of $NADH$ and other biomolecules, ultimately reducing $O_{2}$.
- This transports a total of 10 protons out of mitochondrial matrix
- This "recycles" $NADH$ and $QH_{2}$ into their oxidized forms
Summary II
- REDOX centers (Fe-S, hemes, Q, cytochromes) allow ordered, vectorial movement of electrons towards increased reduction potentials
- Integral redox centers couple electron transport to allosteric conformational changes in complexes
- Allosteric conformational changes in complexes lead to transport of protons