Final on Friday, 12/8 at 10:15 am in MCK 229 (this room)
Final is cumulative
Review session on Thursday, 12/7 at 9:00 am in Allen 141
Photosynthesis I
2017-11-29
Conceptual goals
- Understand the mechanism of the photosynthesis light reactions
- Understand the energetics of the photosynthesis light reactions
Skill goals
- Reason about energy transduction
Photosynthesis is the source of (most) energy in the biosphere
It creates the building blocks from which everything else is made
Photosynthesis has two main parts
- "Light" reactions (today). Capture energy from light and use it to make $ATP$ and $NADPH$
- Carbon fixation (sometimes called "dark") reactions (next class). Reduce $CO_{2}$ to create sugars
In plants, photosynthesis occurs in chloroplasts
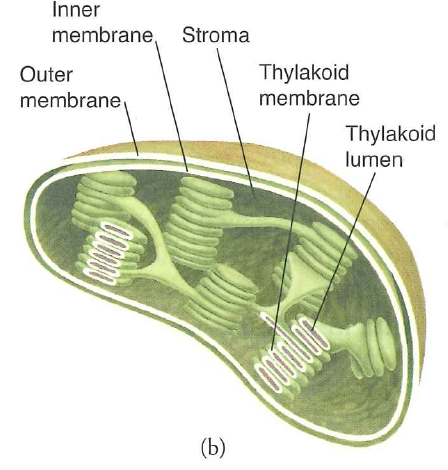
 Credit: wikipedia
Credit: wikipedia
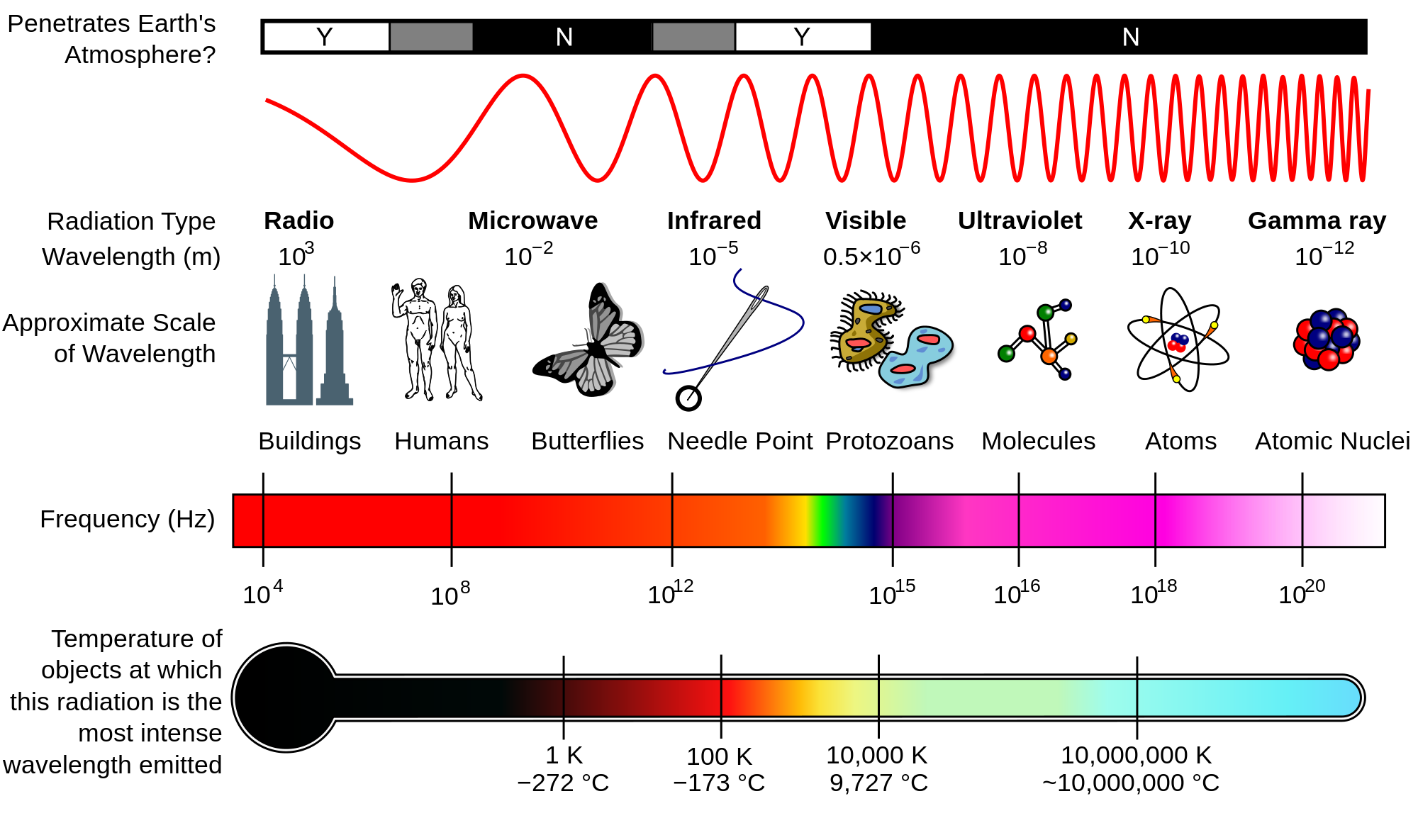
The bluer the light, the higher the energy
A photon's energy is inversely proportional to wavelength
$E = \frac{hc}{\lambda}$
$E$: energy; $h$: Planck's constant; $c$: speed of light; $\lambda$: wavelength
When photons are absorbed, they move electrons from the ground to excited state
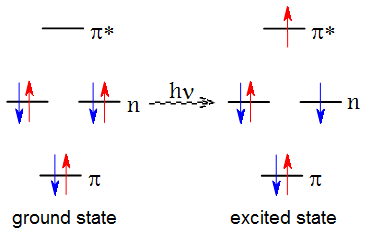
Credit: UCSB
Energy of photon must match energy gap between ground and excited states
What are the possible fates of the excited electron?
- Lost as heat
- Re-emitted as a photon (fluorescence, phosphorescence)
- Directly excites an electron in another molecule (exciton transfer)
- Electron is lost to another molecule (photooxidation)
Photosynthetic molecules are tuned for exciton transfer and photooxidation
Photosynthesis relies on conjugated molecules with extensive $\pi$ orbitals
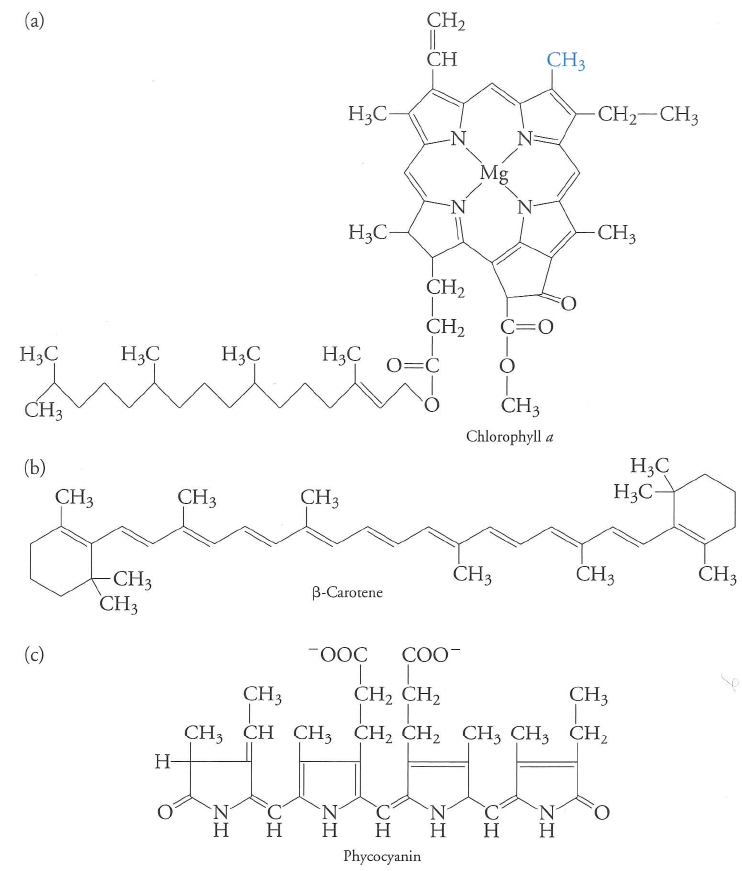
Different molecules have different absorption maxima
Plants appear green because these pigments absorb strongly in the blue and red regions but reflect green
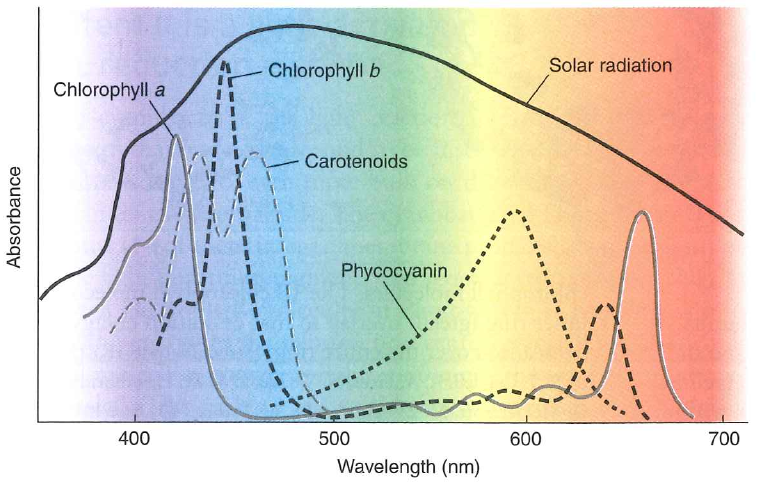
So why don't they have a pigment that absorbs all of that green light?
Not a chemical limit, a physiological one. Would absorb too much heat.
Pigments are exquisitely arranged to maximize absorption and exciton transfer
Pigments are exquisitely arranged to maximize absorption and exciton transfer
Pigments are exquisitely arranged to maximize absorption and exciton transfer
Light harvesting complexes funnel energy to reaction centers by exciton transfer
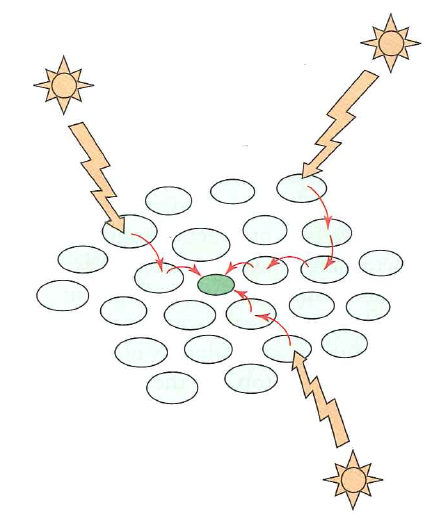
Why might it be helpful to have multiple pigments funneling into a single reaction center?
- Allows efficient capture over a large area
- Allows capture of multiple wavelengths
Okay, so we can capture a photon... now what?
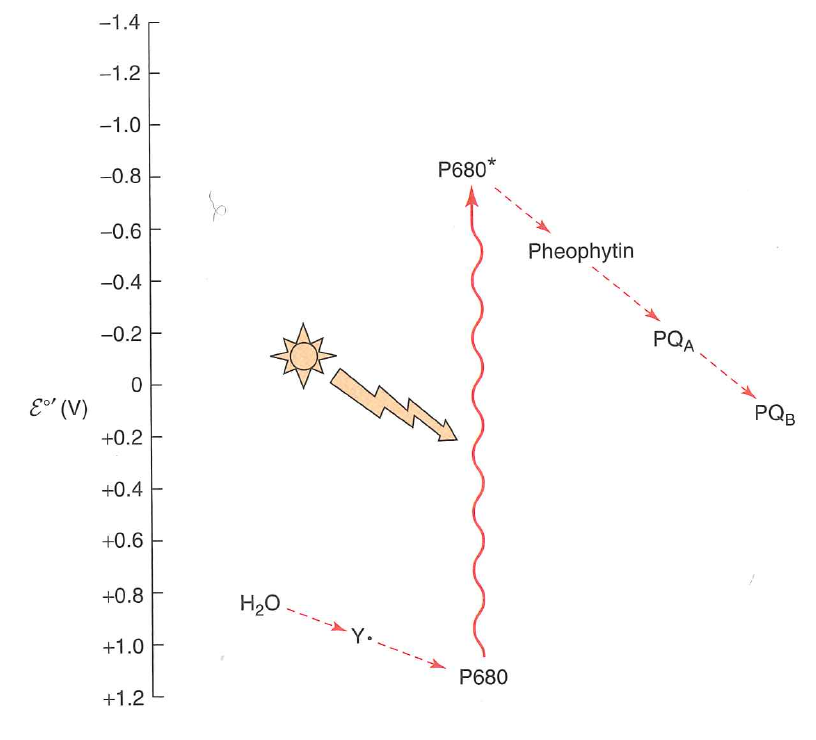
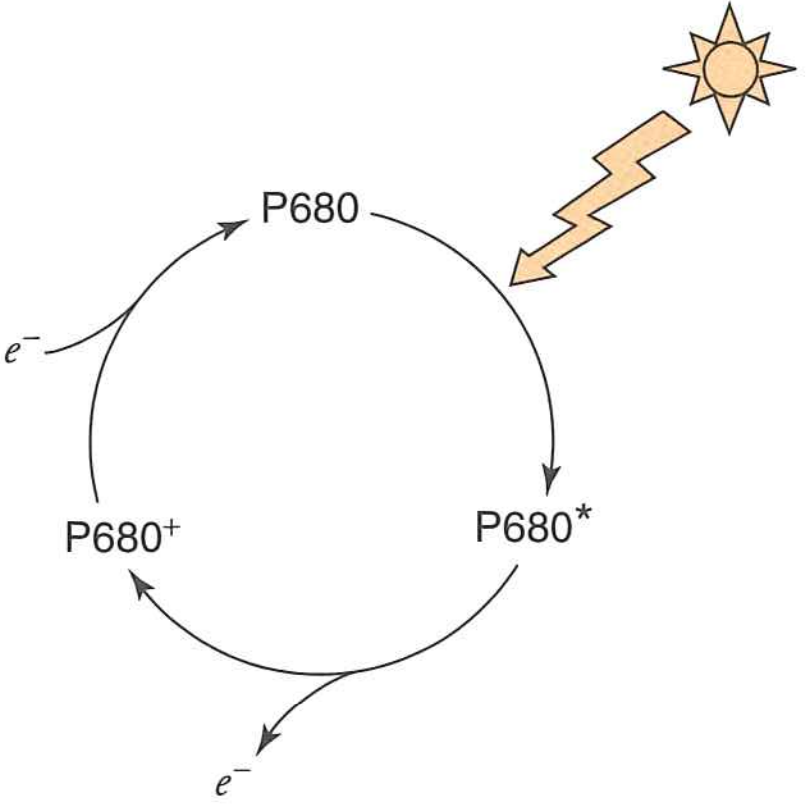
The energy from the photon is transferred to $P680$ which enters its excited state $P680^{*}$
$P680^{*}$ has $\varepsilon^{\circ \prime} = -0.8\ V$
Put another way, an easy way to relax the system is simply to lose the exicted electron
Way lower affinity than $NAD^{+} + H^{-} \rightarrow NADH$ ($-0.32 \ V$)
What do you think happens next?
Electron transport, just as we saw in oxidative phosphorylation!
This electron is transferred to plastiquinone
plastiquinone (photosynthesis)
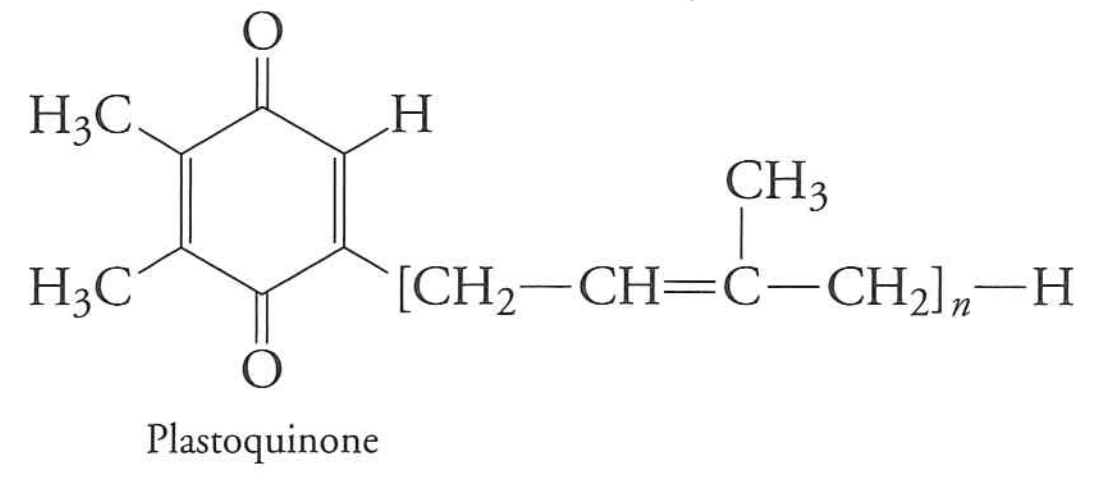
Ubiquinone (oxidative phosphorylation)
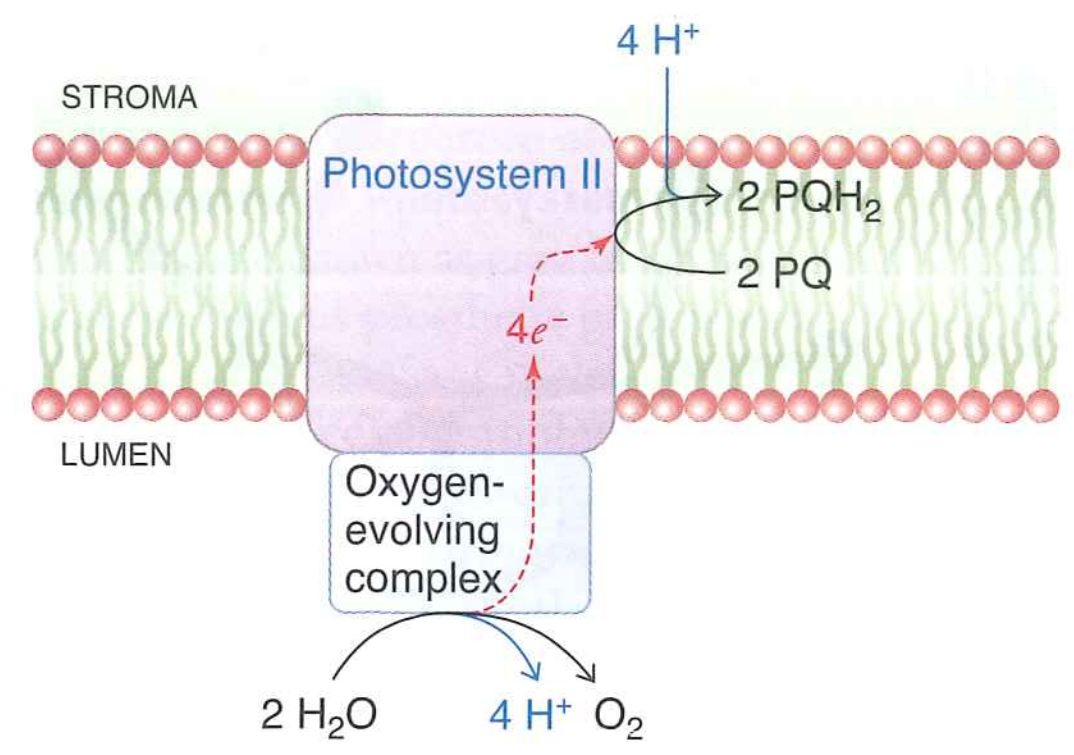
Takes four photons oxidize two $H_{2}O$ into one $O_{2}$
Generates $1O_{2}$, moves (net) $4H^{+}$ into the lumen, and reduces $2PQ \rightarrow 2PQH_{2}$
What happened to the $P680^{*}$?
When it loses its electron, it is now $P680^{+}$ and has extremely high $e^{-}$ affinity
It can scavenge another $e^{-}$ from $H_{2}O$.
$P680^{+}$ oxidizes $H_{2}O$: it is a better oxidant than $O_{2}$!
Pulls electrons off one by one, catalyzed using a $Mn_{4}CaO{5}$ cluster
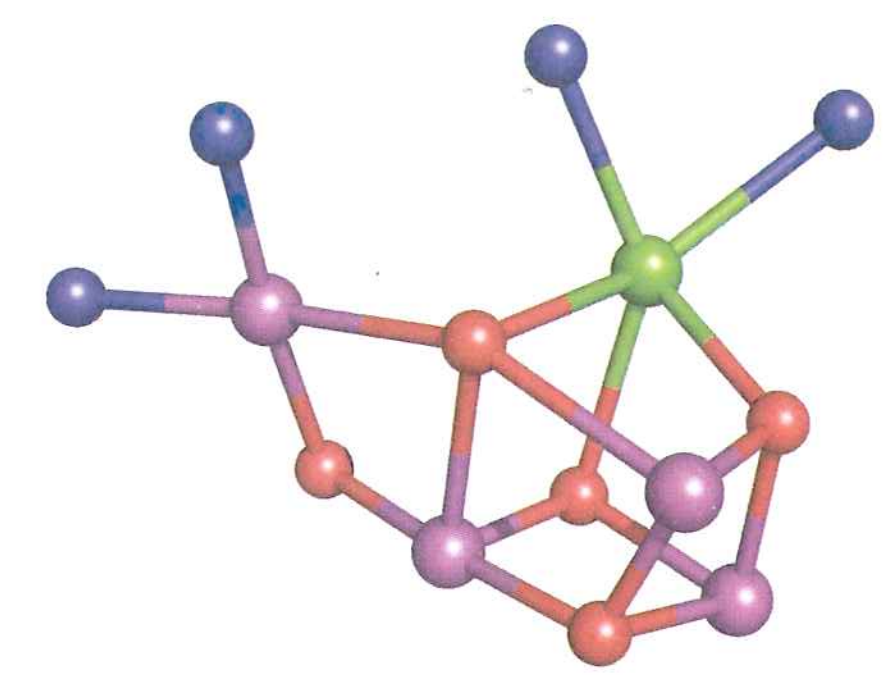
Now what? How do we get an electron off of something that has such a strong electron affinity ($\varepsilon^{\circ \prime} = 1.15\ V$)?
Photooxidation: photon kicks $e^{-}$ into exicted state where it is easily lost.

What happens to the electrons that were on $PQH_{2}$?
Transfer through another series of steps...
Another photooxidation event is used to reduce $NADP^{+}$ to $NADPH$
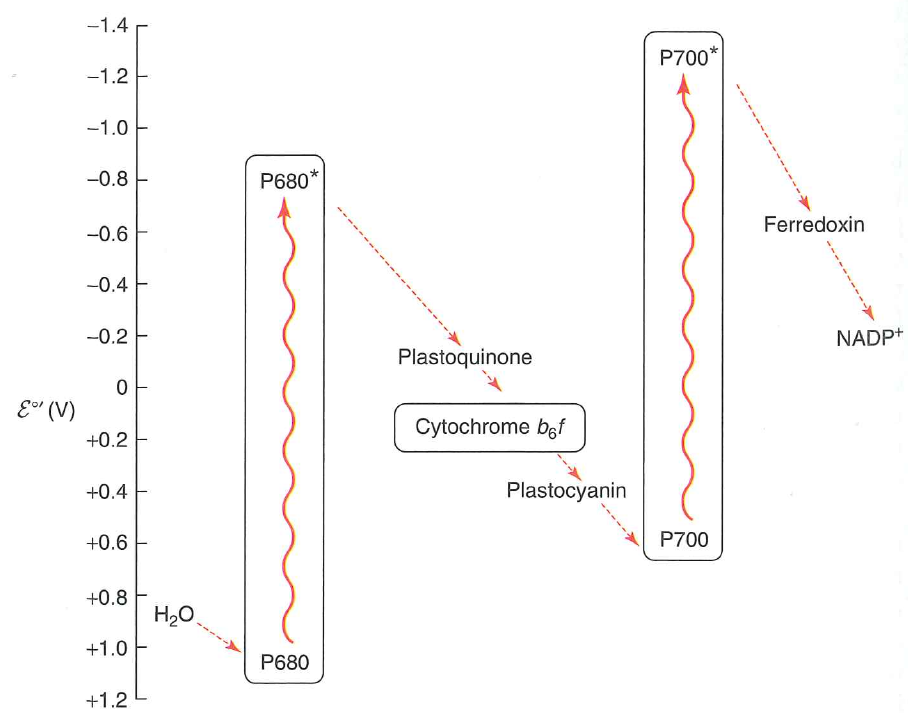
$NADPH$ is used extensively for anabolic processes downstream
Total summary of photooxidation
- PSII:
- $4\ photons$
- $2\ H_{2}O \rightarrow 4H^{+} + O_{2}$
- $2\ PQ + 4H^{+} \rightarrow 2PQH_{2}$
- PSI:
- $4\ photons$
- $2PQH_{2} \rightarrow 2\ PQ + 4H^{+}$
- $2\ NADP^{+} + 2H^{+} \rightarrow 2NADPH$
Over this process, roughly 12 protons are transported from the stroma to the lumen
What happens to these protons?
They are used to make ATP using ATP synthase
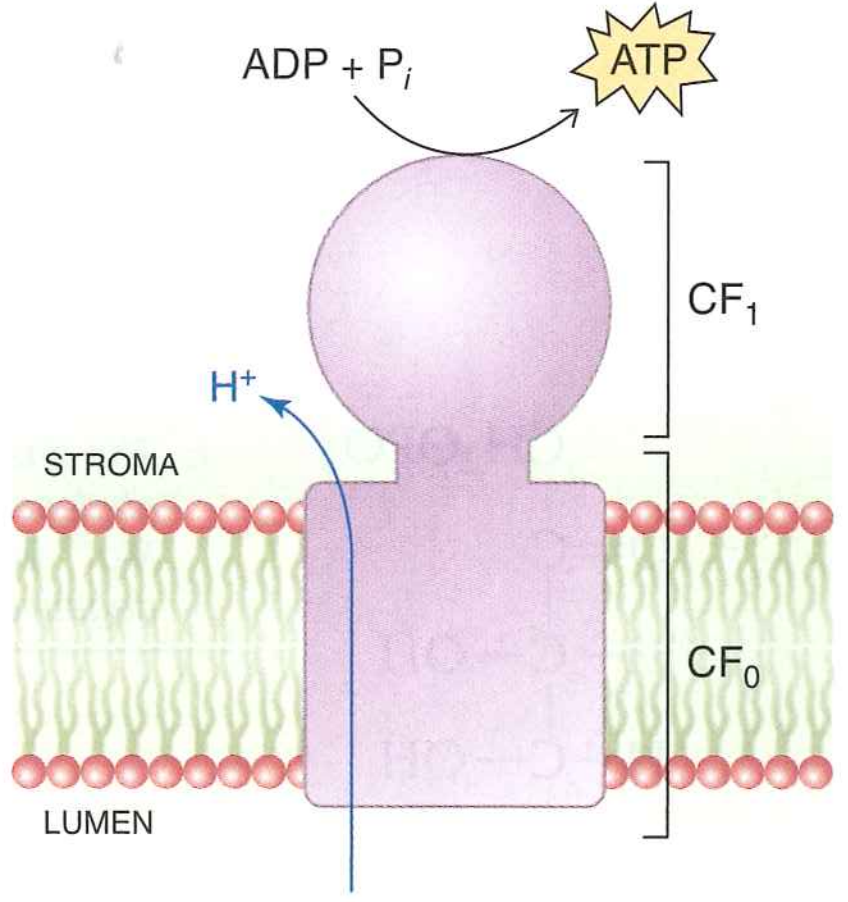
Summary of events
- Energy from photons is used to photooxidize $P680$, meaning it loses a high-energy electron
- It reacquires in electron by oxidizing $H_{2}O$ into $O_{2}$
- $P680$ electrons reduce $PQ$ to $PQH_{2}$ (almost exactly like $Q$ to $QH_{2}$ in respiration)
- A second photon absorption (ultimately) reduces $NADP^{+}$ to $NADPH$
- Just like in respiration, these redox events are coupled to transport of protons across the bilayer
- This proton gradient is then converted to $ATP$ by ATP synthase
Key point:
Photophosphorylation is highly similar to oxidative phosphorylation, except high energy electrons come from excited $P680^{*}$ rather than glucose
Outputs of light reactions are $ATP$ and $NADPH$
$NADP^{+}$ is the terminal electron acceptor (analagous to $O_{2}$ in respiration)