Food is going to kill you
2016-11-22
Conceptual goals
- Understand metabolism in (slightly) broader context
- Understand how finely balanced biochemistry is...it's a miracle we're alive at all
Using oxygen is a dangerous business
Cyanobacteria evolved about 2.1 billion years ago
They brought some new-fangled chemistry: photosynthesis
This was (arguably) the worst pollution event in history. "Green" chemistry is dangerous chemistry.
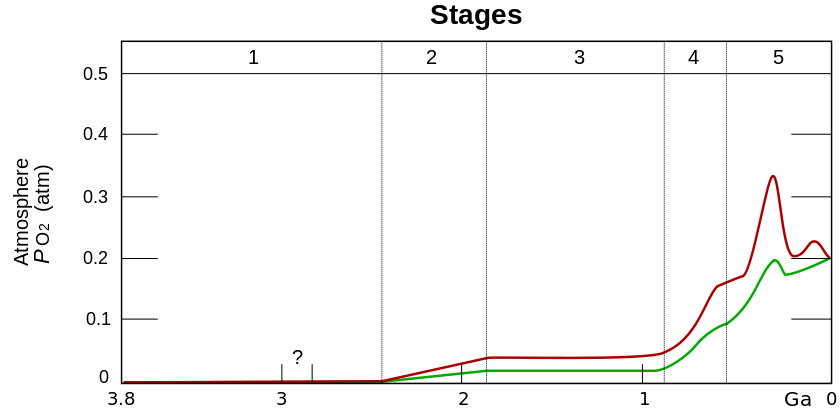
Credit: Protist Info ServerThe evolution of photosynthesis led to accumulation of $O_{2}$ in the atmosphere for the first time
Credit: wikimedia.org
Accumulating $O_{2}$ had several effects:
- It killed a huge number of species (by mechanisms we'll talk about)
- It formed a huge amount of insoluble iron oxides ($Fe_{3}O_{4}$ and $Fe_{2}O_{3}$).
"Iron band" formations around the world help date these events

wikimedia.org
Accumulating $O_{2}$ had several effects:
- It killed a huge number of species (by mechanisms we'll talk about)
- It formed a huge amount of insoluble iron oxides ($Fe_{3}O_{4}$ and $Fe_{2}O_{3}$).
- It reacted with methane ($CH_{4}$) in the atmosphere to form $CO_{2}$ and $H_{2}O$. This destroyed the methane "greenhouse" and probably gave a snowball earth: a global ice age
- It made complex life possible by giving a terminal $e^{-}$ acceptor for respiration
The big problem (and power) of oxygen: it has really high affinity for electrons.
Is this a high or low $\varepsilon^{\circ \prime}$? HIGH. $\frac{1}{2}O_{2} + 2H^{+} + 2e^{-} \rightarrow H_{2}O$ has $\varepsilon^{\circ \prime} = 0.815\ V$
Why does this high reduction potential allow $O_{2}$-using creatures to extract more energy from sugar than they could without $O_{2}$?
$O_{2}$ provides a "sink" for electrons that drives a highly favorable set of oxidation reactions. Anaerobic respiration is limited by the electron affinity of its terminal sink (i.e. pyruvate to lactate at $-0.185\ V)$
Unfortunately, this energy is dangerous to extract...
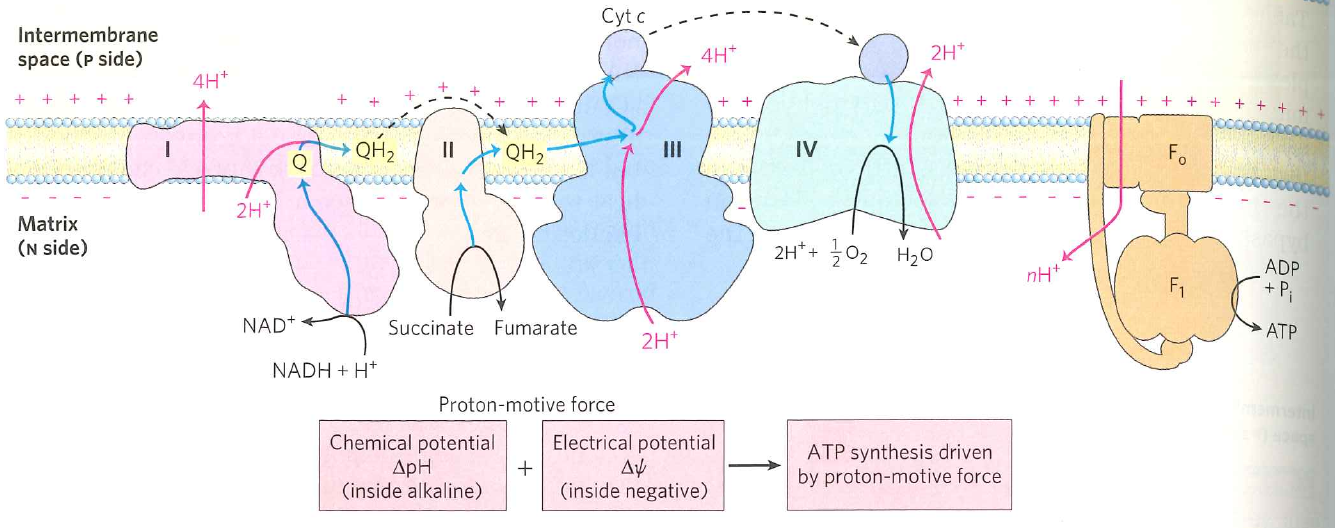
What happens if you run out of $ADP$ (and why)?
- ATP synthesis is the only way to relieve the proton gradient.
- Gradient becomes so large that electron transport can't pump against gradient.
- Electrons accumulate in chain.
- Bad things happen
Big problem points are handoffs involving $Q$
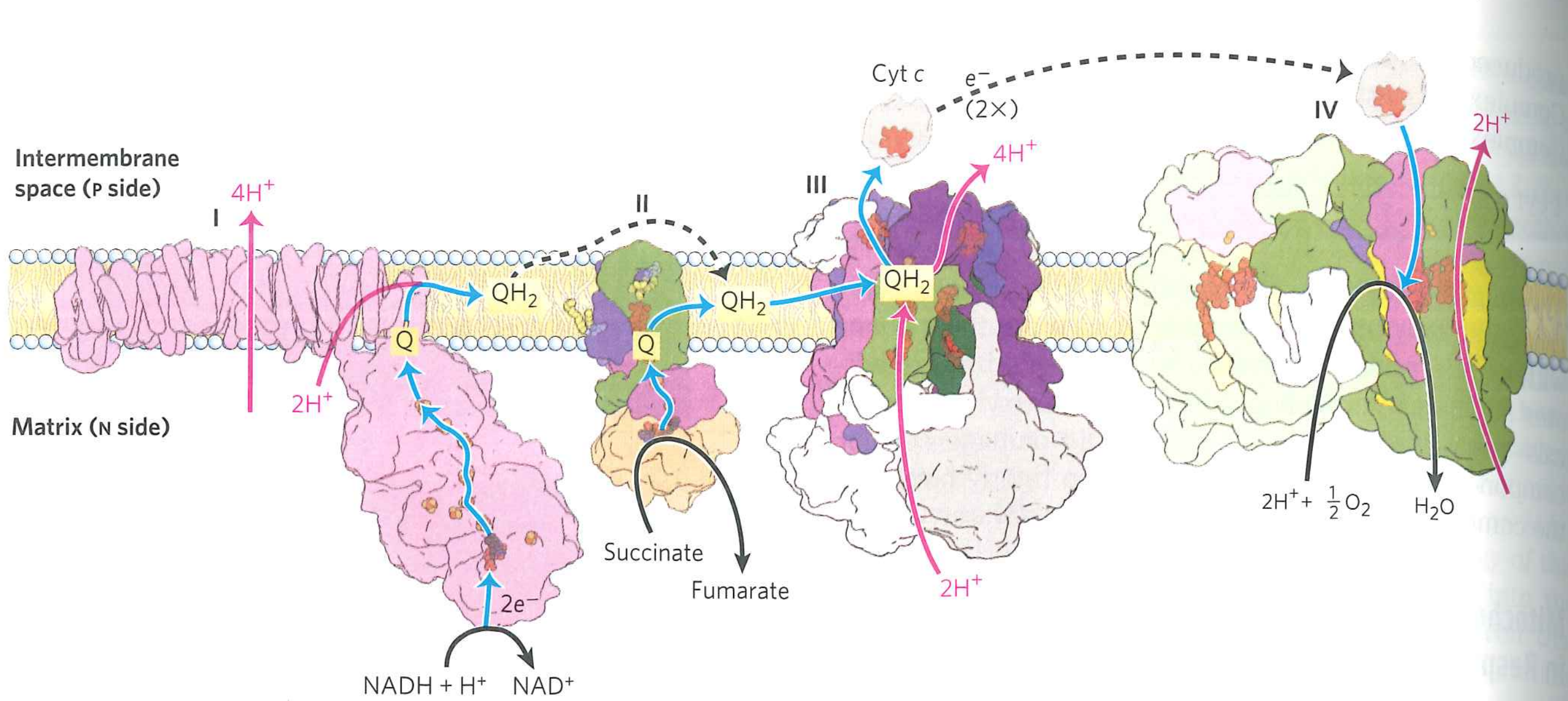
$QH_{2}$ carries $2e^{-}$, while all of the reaction centers carry only $1e^{-}$. ${\cdot}Q^{-}$ is generated as an intermediate
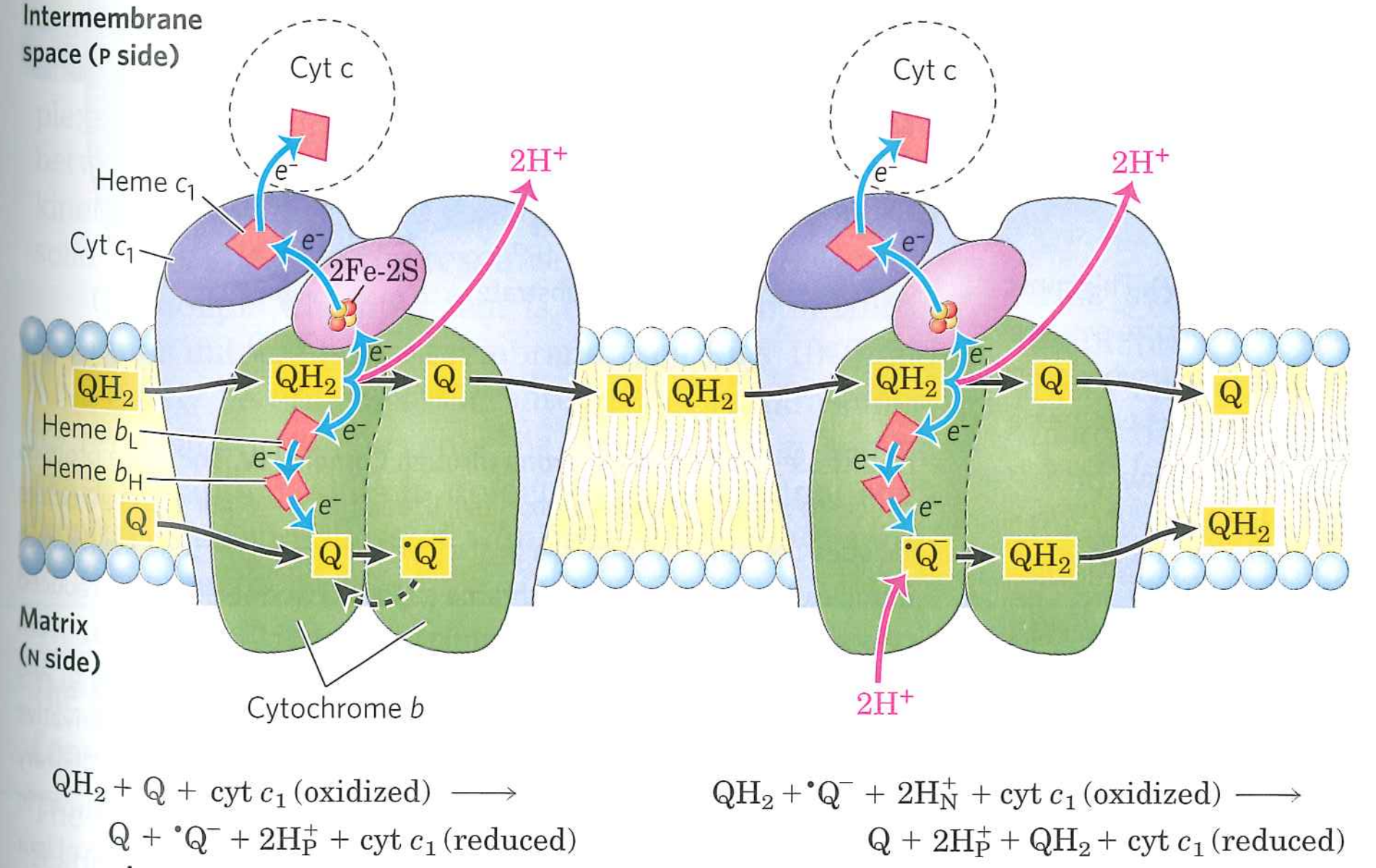
In actively respiring mitochondria, up to 4% of $O_{2}$ is converted to superoxide by errant $\cdot Q^{-}$.
$\cdot Q^{-} + O_{2} \rightarrow Q + \cdot O^{-}_{2}$
superoxide is highly reactive and can go on to modify enzymes, lipids, and nucleic acids
This is very bad
Example: in the lab, you treat an enzyme with $\cdot O^{-}$ and then measure it's enzyme kinetics. $K_{M}$ is unchanged, but $V_{max}$ is much lower.
What might have happened?
- Maybe it reacted at active site (suicide inhibitor)
- Maybe it modified a site distant from the active site (allostery)
These sorts of modifications disrupt protein function and thus living
Cells have defenses against superoxide:
- Superoxide dismutase catalyzes ${\cdot}O^{-}_{2} + {\cdot}O^{-}_{2} + 2H^{+} \rightarrow H_{2}O_{2} + O_{2}$
- Cells use $NADPH \rightarrow NADP^{+} + 2e^{-} + H^{+}$ to reduce $H_{2}O_{2}$ to $H_{2}O$
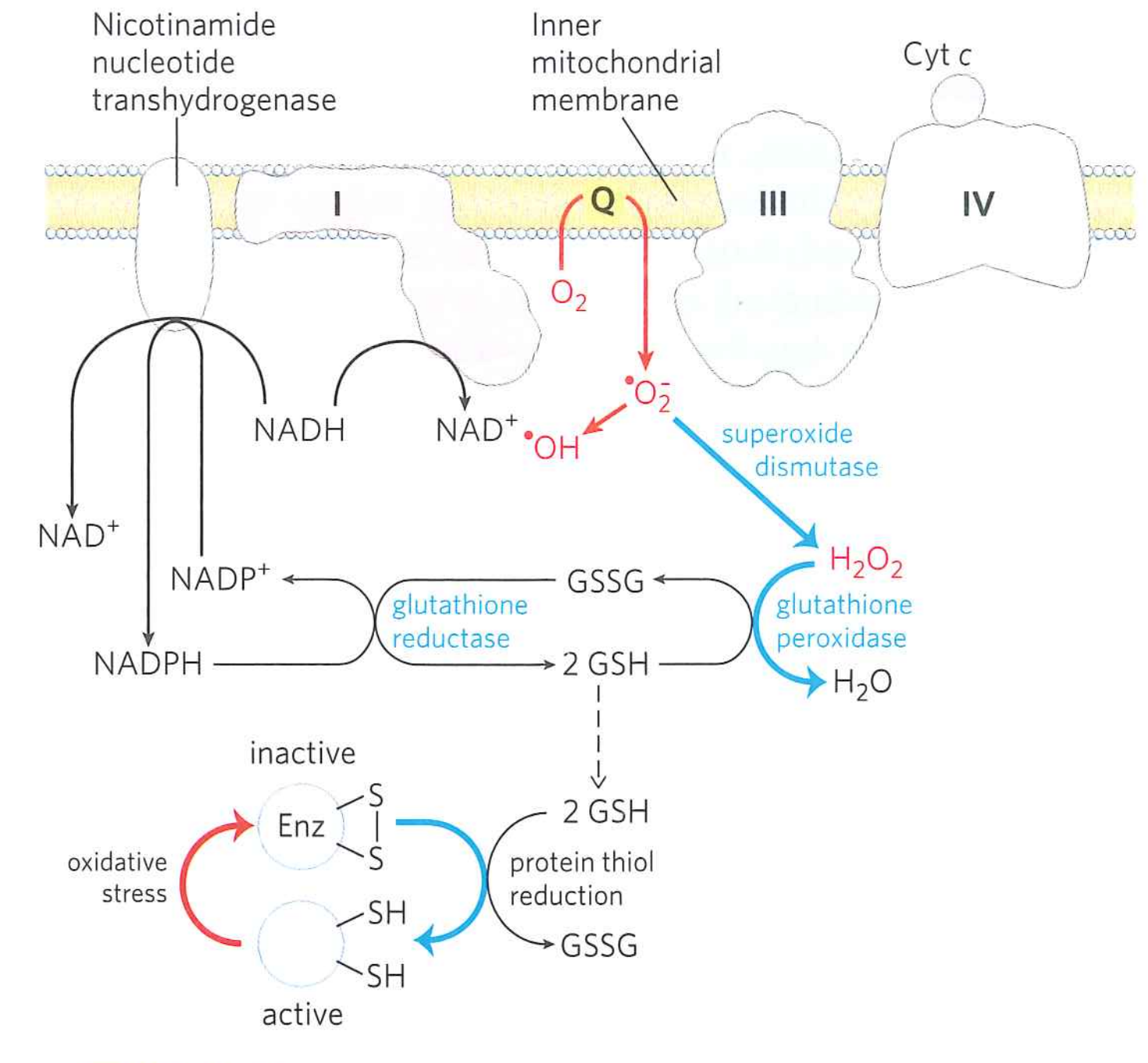
But it's not enough. Damage will accumulate.
Damaged DNA leads to cancer
Oxidized lipids form the nucleus for plaque formation in arteriosclerosis
Along with other forms of damage
DNA: Cells have a huge number of mechanisms to repair DNA damage
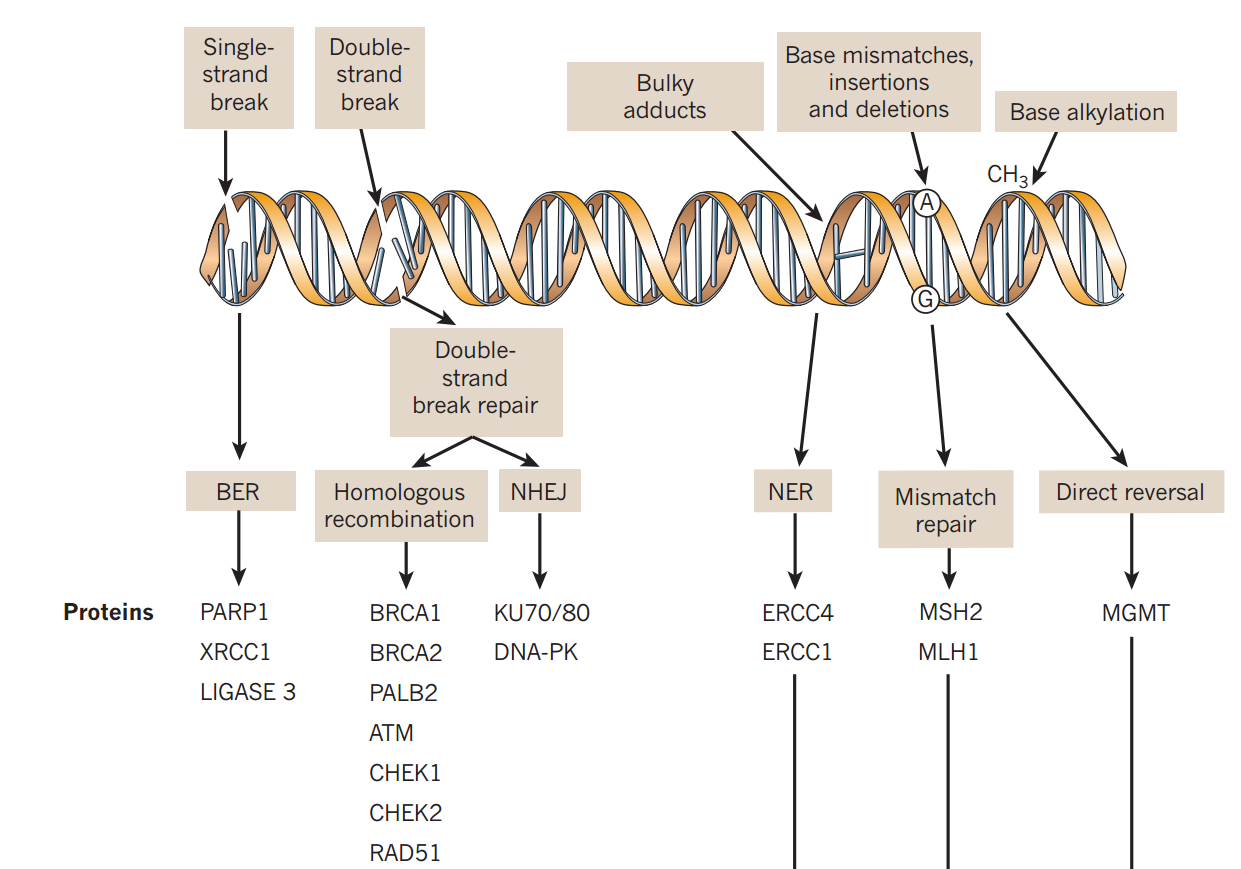
Lord & Ashworth (2012) Nature 481:287-294
But it's still not enough.
Humans have ~40 trillion cells. Over a long enough time, damage will leak through all of these mechanisms
Damaged DNA can disrupt regulation of gene expression that controls cell growth.
This leads to an out-of-control cell that then passes on its out-of-controllness to it's progeny: cancer
High fat + oxidative stress leads to plaque formation, nucleated by oxidized lipids
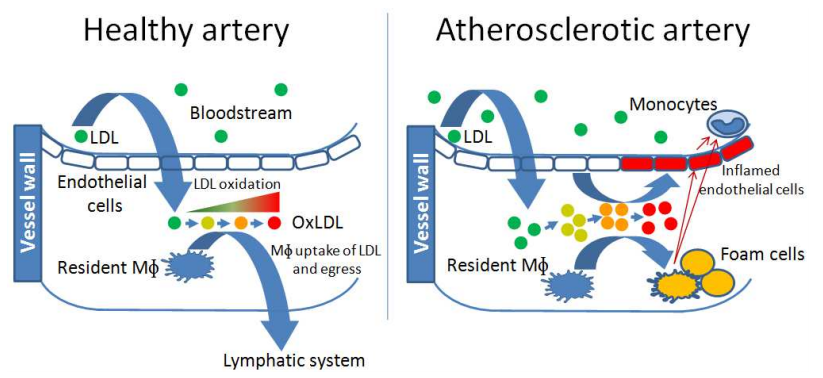
Source: Garelnabi (2012)
And what's one way to get a lot of fat made?
Eat lots of fructose
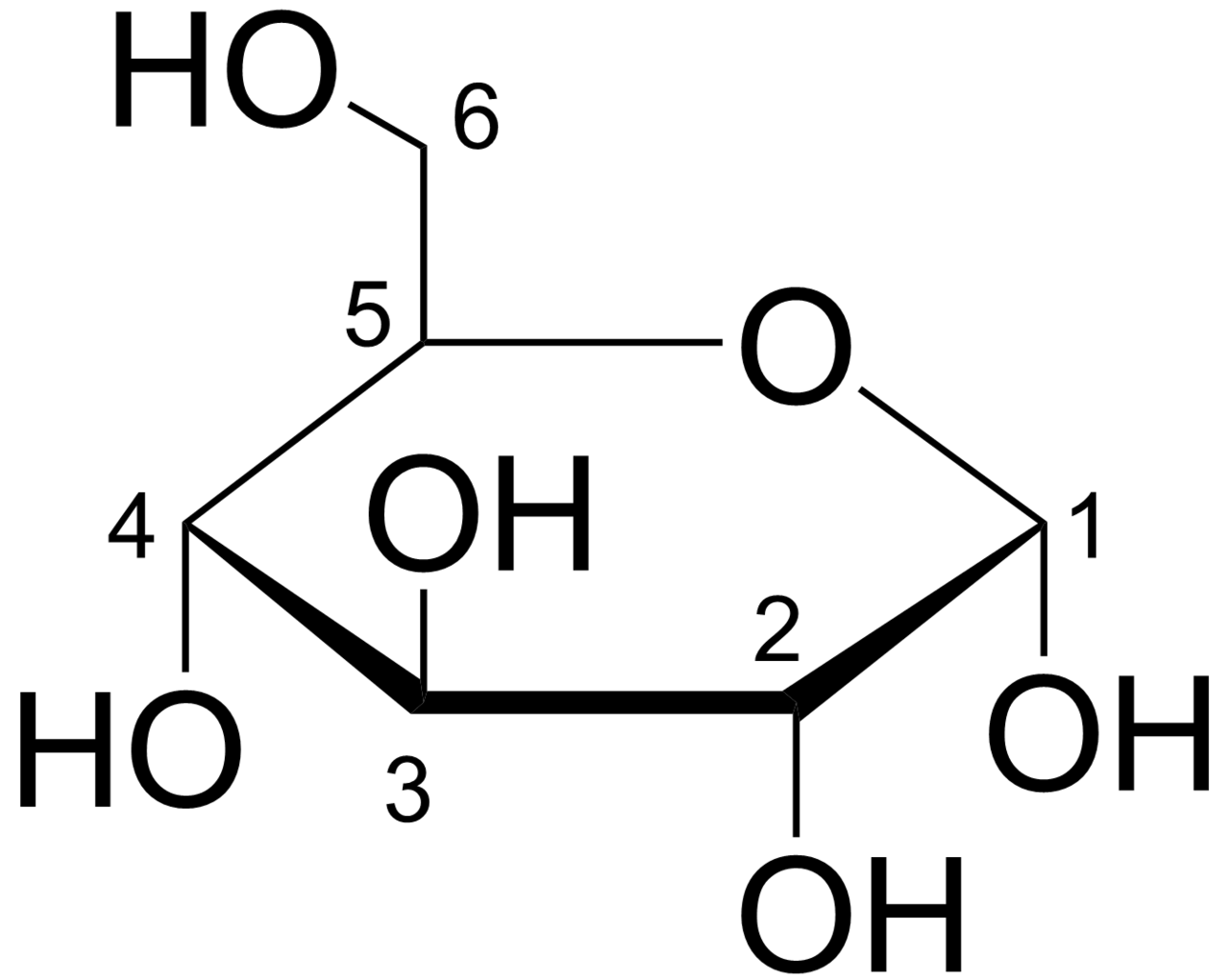
Fructose looks a lot like glucose
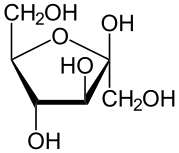
But it's processed differently.
Consumption of fructose has gone way up, concomitant with increase in obesity
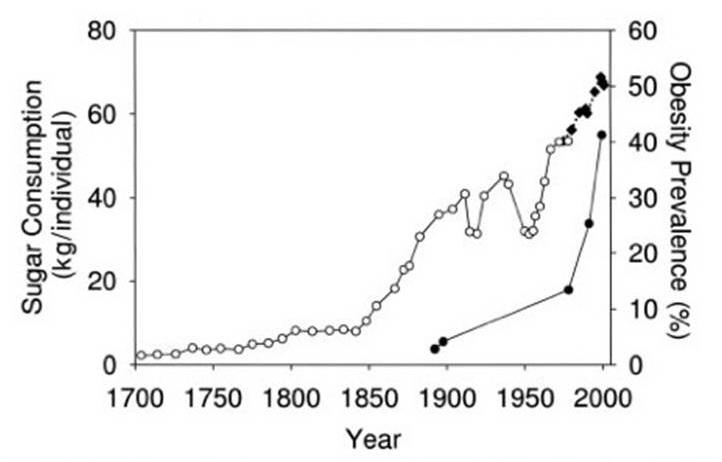
Glucose is absorbed and processed by many tissues, but fructose is primarily absorbed in the liver
The liver expresses the enzyme fructokinase, which catalyzes the formation of fructose-1-phosphate
F1P cannot leave the cell because of it's charge, so it accumulates in the liver
F1P can be converted to DHAP and GA3P (of glycolysis fame)
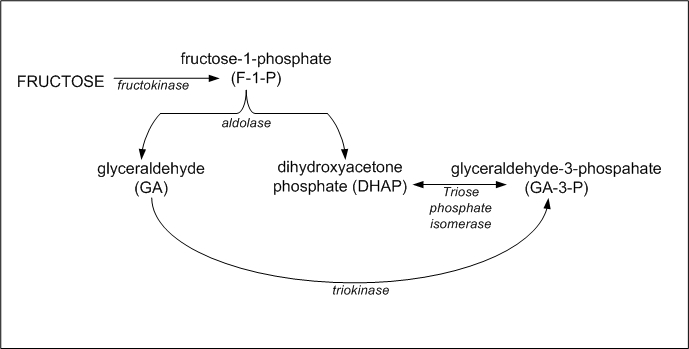
BUT, in doing so, it bypasses phospho-fructokinase
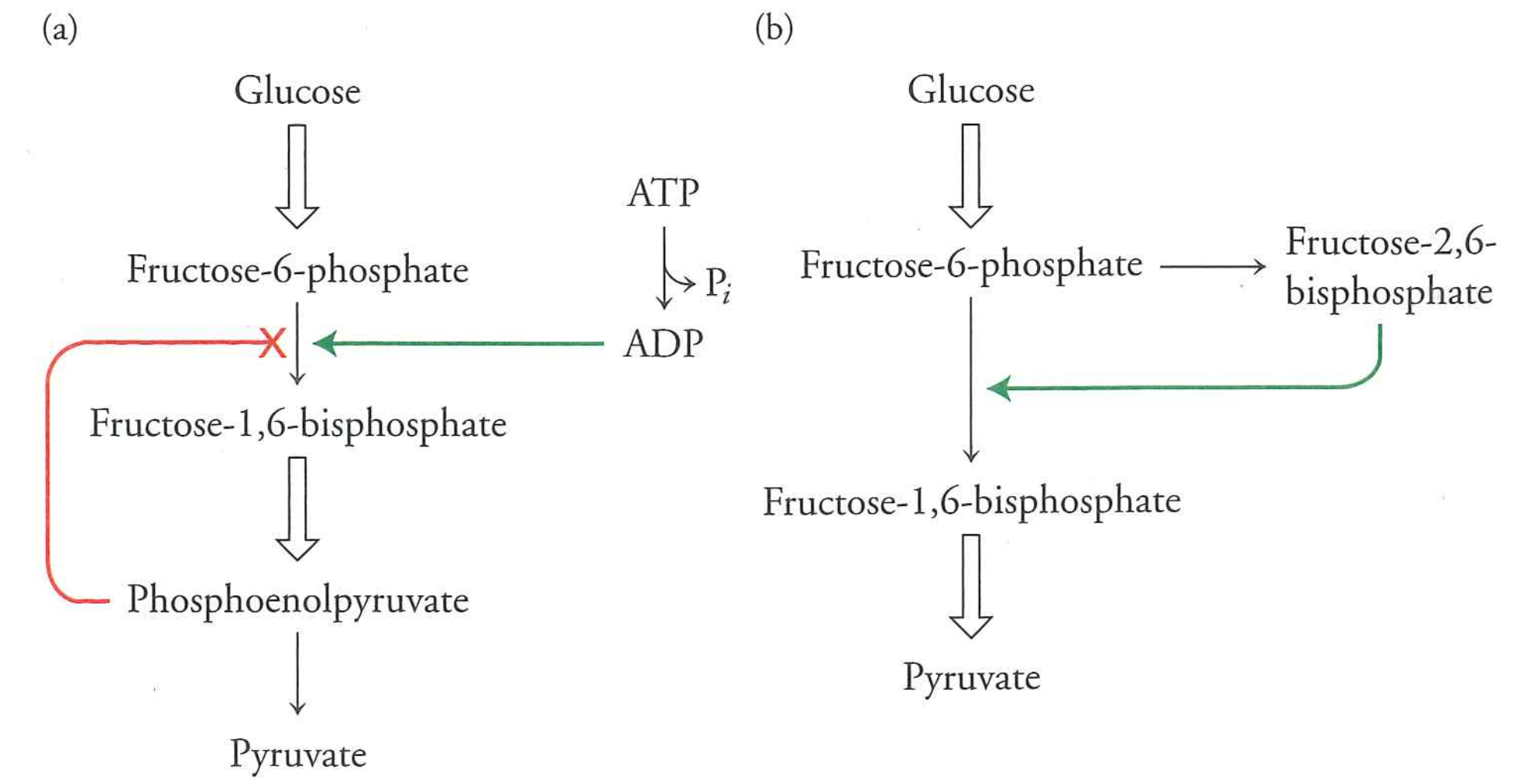
When you eat fructose, it's sent down the glycolysis pipe, even if there's plenty of energy around
A large excess of energy rich molecules triggers fat production
Worse, F1P can feed directly into fat production pathways
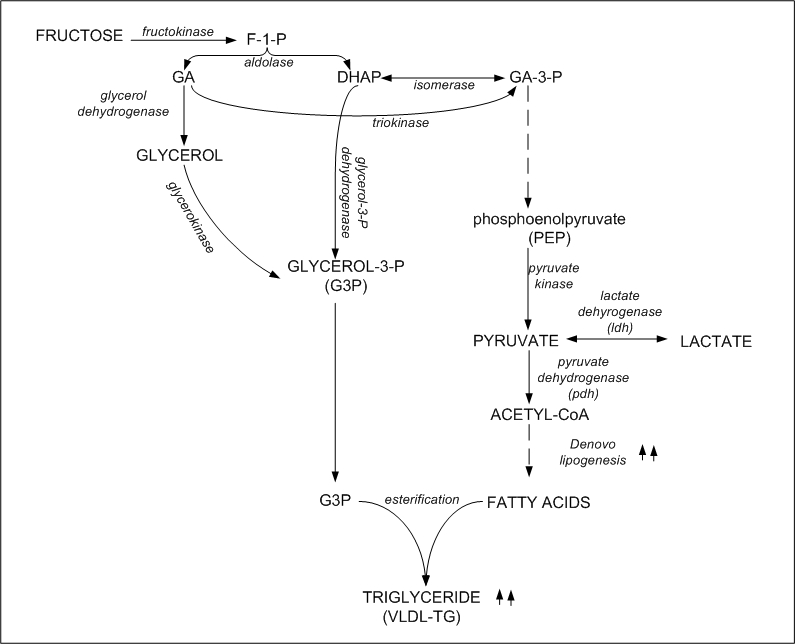
- Fructose is burned directly because it skips PFK, leading to a glut of energy and turing on fat production
- Fructose itself is an efficient substrate for making fat
- This happens preferentially in the liver, leading to fatty liver (which is a central regulator of metabolism in mammals, leading to even worse outcomes over time)
- Oxidative stress plus high fat leads to arterial plaques, heart disease, stroke, etc.
Exercise:
Hexokinase can make F6P rather than F1P, which would solve all of these problems. Why doesn't this happen?
Hexokinase/fructose $K_{M} = 12 \ mM$; $k_{cat} = 20\ s^{-1}$
fructokinase/fructose $K_{M} = 0.5 \ mM$; $k_{cat} = 8\ s^{-1}$
If both proteins have the same concentration, how much faster will fructokinase operate on 0.1 mM fructose?
Summary:
- $O_{2}$ is vital to respiration, but also dangerous.
- It can form superoxide, which damages molecules
- Damaged proteins lead to poor cellular function, DNA to cancer, and lipids to heart disease
- Fructose skips the key regulatory step in glycolysis, leading to fat production and heart disease
